Scientists in Japan have demonstrated how a drug that is currently used to treat high blood pressure, activates a mitochondrial stress response to prolong lifespan in the roundworm, Caenorhabditis elegans. The team, led by Eriko Kage-Nakadai, PhD, at Osaka City University in Japan, says the results of their studies provide new insights for continuing anti-aging research, and could point to drug repurposing as a strategy for controlling the aging process. Kage-Nakadai commented, “What is particularly exciting is that we tested already available approved drugs here, and we have revealed the potential of repurposing existing drugs for aging control. Worms always give us many hints.”
The researchers reported on their work in Biogerontology, in a paper titled, “Metolazone upregulates mitochondrial chaperones and extends lifespan in Caenorhabditis elegans.”
People have always been fascinated by the potential to stop the aging process, and nearly every culture has stories to tell about people who lived for thousands of years. While scientists today aren’t looking for any fabled fountain of youth, the potential to promote longevity has prompted research into the mechanisms of aging and the possibility of developing anti-aging drugs.
Aging is a complex process that involves a deterioration of certain cellular and physiological mechanisms, and researchers suggest that mitochondria play an important role in this aging process. “Among the many contributors to aging, such as genetic predisposition, programmed senescence, and DNA damage, mitochondria have been implicated as one of the key players,” the authors wrote.
Specifically, when mitochondria are damaged and their function is impaired, a transcriptional response called the mitochondrial unfolded protein response (UPRmt) is triggered to repair mitochondria, and so help to improve cell survival. “UPRmt promotes the recovery of mitochondrial fusion and fission, as well as mitochondrial DNA replication, thereby facilitating the survival of the cell,” the team explained. Some scientists think it might be possible to increase lifespan by identifying drugs that activate this UPRmt pathway.
Kage-Nakadai and her team at Osaka City University have a particular interest in aging research. “Even though aging is not a disease, drugs may slow down aging and mitigate or prevent its negative effects on our health,” she said. Current research has also demonstrated promising signs. Experiments with the common research model, C. elegans, have found several compounds that increase the worm’s lifespan by triggering this UPRmt.
Against the backdrop of previous studies, the Kage-Nakadai team screened about 3,000 drugs in worms that had been engineered to glow if the drug treatment activated hsp-6, a gene that is highly expressed when UPRmt occurs. “Nuclear-encoded mitochondrial genes, such as the chaperone HSP-6 (mtHSP70 in mammals), are induced during the UPRmt in C. elegans,” they noted. Of the compounds screened, 1,300 were off-patent drugs approved by various global regulators. The remaining 1,700, were unapproved bioactive molecules.
The team’s screen highlighted metolazone, a diuretic drug that is used to treat heart failure and high blood pressure. When the researchers then tested the drug on wild-type C. elegans, they found that the lifespan-extending effect was comparable to that of another drug, AZD281 (olaparib), which has previously been shown to extend the lifespan of C. elegans via the UPRmt, and which was used as a positive control.
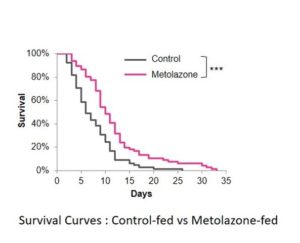
The researchers’ experiments also showed that metolazone induced hsp-6 (Hspa9 in humans) expression in human HeLa cells, suggesting that its UPRmt-related effects could possibly span multiple species. “In human cells, supplementation with metolazone induced the expression of mtHSP70 and HSP60, which encode mitochondrial chaperones: however, ER chaperone HSP70 was not induced,” they further commented. “Taken together, we conclude that metolazone is a potent and specific mitochondrial chaperone activator across species.”
The researchers acknowledged that their reported work is still in the early stage, but it does open up a potential new route for developing future anti-aging drugs. “In conclusion, the present study shows that the antihypertensive drug, metolazone, activates the UPRmt across species and promotes lifespan extension in C. elegans,” they wrote. “Our findings may be useful in devising a strategy to develop anti-aging drugs based on drug repurposing and present a clearer understanding of the relationship between the UPRmt and longevity.”






