Protein clumps accumulate until they obstruct physiological function. It’s a common theme in neurology, where clumps of β-amyloid are the hallmark of Alzheimer’s disease, just as clumps of α-synuclein are the hallmark of Parkinson’s disease. Now protein clumps are being recognized in cardiology. Specifically, clumps of desmin, a muscle-specific protein that forms support structures in heart cells, appear to be more common in heart failure patients than those without heart failure.
The form of desmin that tends to clump has been identified by researchers from Johns Hopkins University. This knowledge, the researchers indicate, could lead to new diagnostic and prognostic tools that could assess the effectiveness of lifestyle or medical interventions.
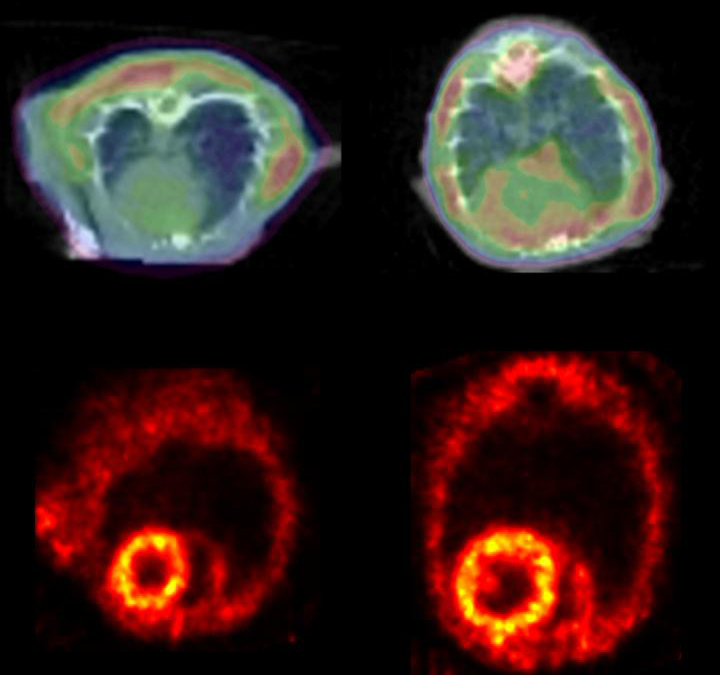
Detailed findings will appear May 11 in the journal Circulation Research, in an article entitled “Desmin Phosphorylation Triggers Preamyloid Oligomers Formation and Myocyte Dysfunction in Acquired Heart Failure.” According to this article, keeping track of desmin post-translational processing and misfolding may help clinicians detect the cardiac accumulation of toxic preamyloid oligomers (PAOs). Myocardial PAOs in acquired forms of heart failure, the article indicates, can be detected via positron emission tomography (PET).
“PET imaging of protein clumps may be eventually used in patients to identify structural changes in the heart as the disease progresses, and this information likely holds prognostic value,” says Peter Rainer, M.D., Ph.D., the article’s lead author and a former postdoctoral fellow at Johns Hopkins. (He is now at the Medical University of Graz in Austria.) “It could be used as a nice measure of the effect of an intervention to halt or reverse disease progression.”
Previous work by the Johns Hopkins team showed that desmin forms clumps in the hearts of dogs with heart failure. In the new study, the team used a common mouse model of heart failure to look for desmin clumps. The team also scrutinized biopsies from human hearts for the presence of desmin protein clumps. To do so, they used a fluorescent antibody commonly used in Alzheimer's disease research and a new fluorescent stain for amyloid developed by Giulio Agnetti, Ph.D., assistant professor of medicine at the Johns Hopkins University School of Medicine.
“[We] show that toxic cardiac PAOs accumulate in the myocardium of mice subjected to transverse aortic constriction (TAC), and that PAOs co-migrate with the cytoskeletal protein desmin in this well-established model of acquired HF [heart failure],” the authors of the article write. “We confirm this evidence in cardiac extracts from human ischemic and non-ischemic HF.”
The researchers found twice as many desmin clumps in heart failure patients than those without heart failure.
Then the researchers treated proteins from the mice hearts with epigallocatechin gallate (EGCG)—a chemical from green tea known to break up amyloid. The treatment cut by half the amount of protein clumps.
“Interestingly, green tea has already been demonstrated to curb the incidence of cardiovascular disease as well as improve cognitive impairment in Alzheimer's models, though the mechanism for such action is unclear,” says Dr. Agnetti. “EGCG's ability to 'de-clump' these sticky proteins could be one of green tea's healthy effects. Knowing how this chemical works could open new avenues for designing a new class of drugs that target protein clumping.”
To identify the form of desmin that tends to clump, the researchers genetically engineered versions of desmin, tagged them with a green fluorescent signal to make them visible, and put them in heart cells using a virus. These desmin forms differed with respect to their phosphate groups, which the researchers found suspect on the basis of previous studies. Ultimately, the researchers demonstrated that “Ser-31 phosphorylated (pSer31) desmin aggregates extensively in cultured cardiomyocytes.”
Finally, the researchers tested if they could use this noninvasive technique to detect desmin clumps in mice with heart failure. Healthy and heart failure mice were injected with Amyvid, a radioactive dye that allows the researchers to see the protein clumps by PET. The heart failure mice had 13% more of the Amyvid taken up in their hearts than the healthy mice.
In future experiments, the research team plans to confirm its results in more human tissue samples. The investigators also hope to identify a drug or small molecule to prevent desmin from forming clumps.
“There is a lot of emphasis placed on the role of genes in modern times, but we're born with our genes and at present we can do very little about the ones we have,” notes Dr. Agnetti. “I think the next step is to follow up with the proteins that are dynamically modified in response to environment, which places a larger emphasis on lifestyle intervention to help prevent diseases. Natural compounds like EGCG in green tea and modified dietary interventions could play a role in keeping us healthy.”