
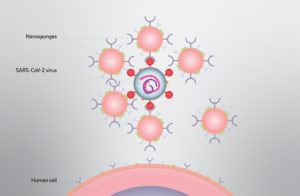
Researchers at the University of California, San Diego (UCSD) have developed “nanosponges” that can attract and neutralize SARS-CoV-2 in cell culture, causing the virus to lose its ability to hijack host cells and reproduce. Cloaked in the cell membranes from either human lung cells or human immune cells, the nanoparticles are designed to protect the healthy cells that the virus invades, rather than targeting the virus itself. The approach effectively uses nanoparticles to soak up harmful pathogens and toxins, hence the name nanosponges.
When tested by researchers at Boston University, both the lung cell and immune cell types of nanosponge caused the SARS-CoV-2 virus to lose nearly 90% of its viral infectivity in a dose-dependent manner. Viral infectivity is a measure of the ability of the virus to enter the host cell and exploit its resources to replicate and produce additional infectious viral particles. “Traditionally, drug developers for infectious diseases dive deep on the details of the pathogen in order to find druggable targets,” said Liangfang Zhang, PhD, a nanoengineering professor at the UCSD Jacobs School of Engineering. “Our approach is different. We only need to know what the target cells are. And then we aim to protect the targets by creating biomimetic decoys.”
Zhang and colleagues reported on their technology in Nano Letters, in a paper titled, “Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity.”
Nanoparticles cloaked in human lung cell membranes and human immune cell membranes can attract and neutralize the SARS-CoV-2 virus in cell culture, causing the virus to lose its ability to hijack host cells and reproduce. The UCSD researchers call their nano-scale particles “nanosponges” because they soak up harmful pathogens and toxins. [David Baillot/University of California San Diego]
Scientists searching for new antiviral drugs need to understand the molecular mechanisms of viral infection, but this is a particular challenge with emerging viruses such as SARS-CoV-2, the authors noted. Moreover, antiviral medicines often target a single viral species, and so aren’t applicable to other viral species or families, and they may also become ineffective as the target virus mutates. “Therefore, an effective therapeutic agent to inhibit SARS-CoV-2 infectivity, as well as its potential mutated species, would be a significant game-changer in the battle against this public health crisis,” they continued.
An alternative approach to antiviral drug development would be to focus on affected host cells instead of targeting the causative agent. Zhang’s team developed the cellular nanosponges as a medical countermeasure to the coronavirus, in light of the fact that the infectivity of SARS-CoV-2 relies on its binding with protein receptors—either known or unknown—on the target host cells.
The biomimetic nanosponge platform was first developed by the Zhang lab more than a decade ago, and is being harnessed for a wide range of applications. With the emergence of SARS-CoV-2, the idea of using the nanosponge platform to fight the new virus came to Zhang “almost immediately,” he said.
SARS-CoV-2 commonly infects lung epithelial cells as the first step in COVID-19 infection, so Zhang and his colleagues reasoned that it would make sense to cloak a nanoparticle in fragments of the outer membranes of lung epithelial cells. Because “… the nanosponges display the same receptors that the viruses depend on for cellular entry,” the investigators wrote, the goal was to see if the virus could be tricked into latching on to the membrane proteins on the nanoparticles instead of attaching to the lung cells.
Each COVID-19 nanosponge consists of a polymer core coated in cell membranes extracted from either lung epithelial type II cells or macrophage cells. The researchers hypothesized that by binding to the nanosponges, each of which is a thousand times smaller than the width of a human hair, the coronavirus particles would then be unable to infect their usual cellular targets. Macrophages—which are white blood cells that play a major role in inflammation—are also very active in the lung during the course of a COVID-19, so Zhang and colleagues created a second nanosponge cloaked in macrophage membrane.
“Based upon the current knowledge of SARS-CoV-2, we fabricated two types of cellular nanosponges, human lung epithelial type II cell nanosponge (denoted “Epithelial-NS”) and human macrophage nanosponge (denoted “MΦ-NS”).” The membranes cover the sponges with all the same protein receptors as the cells they impersonate—and this inherently includes whatever receptors SARS-CoV-2 uses to enter cells in the body.
The researchers prepared several different concentrations of nanosponges in solution to test against SARS-CoV-2. They then collaborated with a team at Boston University’s National Emerging Infectious Diseases Laboratories (NEIDL) to perform independent tests that would assess the ability of the nanosponges to block viral infectivity. Scientists at the level 4 biosafety labs, led by Anthony Griffiths, PhD, associate professor of microbiology at Boston University School of Medicine, tested the ability of various concentrations of each nanosponge type to reduce the infectivity of the same strains of SARS-CoV-2 that are being used in other COVID-19 therapeutic and vaccine research.
The results showed that at a concentration of 5 mg/mL, the lung cell membrane-cloaked sponges inhibited 93% of the viral infectivity of SARS-CoV-2. The macrophage-cloaked sponges inhibited 88% of the viral infectivity of SARS-CoV-2. “From the perspective of an immunologist and virologist, the nanosponge platform was immediately appealing as a potential antiviral because of its ability to work against viruses of any kind,” said Anna Honko, PhD, a co-first author on the paper and a research associate professor of microbiology at NEIDL. “This means that as opposed to a drug or antibody that might very specifically block SARS-CoV-2 infection or replication, these cell membrane nanosponges might function in a more holistic manner in treating a broad spectrum of viral infectious diseases. I was optimistically skeptical initially that it would work, and then thrilled once I saw the results and it sunk in what this could mean for therapeutic development as a whole.”
A therapeutic dose of nanosponges might flood the lung with a trillion or more tiny nanosponges that could draw the virus away from healthy cells. Once the virus binds with a nanosponge, “it loses its viability and is not infective anymore, and will be taken up by our own immune cells and digested,” said Zhang.
In addition to the encouraging data on neutralizing the virus in cell culture, the researchers noted that nanosponges cloaked with fragments of the outer membranes of macrophages could also quiet cytokine storms in COVID-19 patients by soaking up inflammatory cytokine proteins that are implicated in some of the most dangerous repercussions of COVID-19, which are driven by the immune response to the infection. “For the treatment of COVID-19, MΦ-NS may have some significant advantages over Epithelial-NS,” the researchers wrote. “The clinical manifestation of COVID-19 is partially driven by direct viral damage but primarily by the immune response to the infection … Specific to COVID-19, MΦ-NS can neutralize the viral activity not only early on to reduce the viral load in the body but also even late in the disease, and it will be able to address the fulminant inflammation associated with COVID-19.” Zhang further commented, “We will see if the macrophage nanosponges can neutralize the excessive amount of these cytokines as well as neutralize the virus.”
The hope is that the nanosponges may also remain effective against mutated strains of the virus. “Another interesting aspect of our approach is that even as SARS-CoV-2 mutates, as long as the virus can still invade the cells we are mimicking, our nanosponge approach should still work. I’m not sure this can be said for some of the vaccines and therapeutics that are currently being developed,” Zhang continued. The researchers envisage that the nanosponges would in addition be effective against any new coronavirus or even other respiratory viruses, including whatever virus might trigger the next respiratory pandemic.
“The nanosponge platform offers a unique benefit over other therapies currently in development for COVID-19 in that the nanosponges are mutation and potentially virus agnostic,” they wrote. “In principle, as long as the target of the virus remains the identified host cell, the nanosponges will be able to neutralize the infection, providing a broad-acting countermeasure resistant to mutations and protection against this and other emerging coronaviruses.”
The UCSD researchers and collaborators aim to evaluate efficacy of the nanosponges in animal models, in the next few months. The team has already shown short-term safety in the respiratory tracts and lungs of mice.
Using macrophage cell fragments as cloaks builds on years of work to develop therapies for sepsis using macrophage nanosponges. In work reported in 2017, Zhang and a team of researchers at UCSD showed that macrophage nanosponges can safely neutralize both endotoxins and proinflammatory cytokines in the bloodstream of mice. Zhang co-founded a San Diego biotechnology company, Cellics Therapeutics, which is working to translate this macrophage nanosponge work into the clinic.
There will be a significant amount of research to be completed before scientists know whether the COVID-19 nanosponge platform would be a safe and effective therapy against the virus in humans, Zhang cautioned. “The utility of the cellular nanosponges for the treatment of SARS-CoV-2 infection requires further validation in appropriate animal models, which is currently underway, and this will pave the way for human clinical trials in the future,” the investigators noted. But if the sponges do reach the clinical trial stage, there are multiple potential ways of delivering the therapy, including direct delivery into the lung for intubated patients, via an inhaler like for asthmatic patients, or intravenously, especially for treating the complications of cytokine storm.
There’s also the potential to use the approach prophylactically. “I see potential for a preventive treatment, for a therapeutic that could be given early because once the nanosponges get in the lung, they can stay in the lung for some time,” Zhang said. “If a virus comes, it could be blocked if there are nanosponges waiting for it.”
The first of nanosponges created by Zhang’s lab were cloaked with fragments of red blood cell membranes. These nanosponges are being developed to treat bacterial pneumonia and have undergone all stages of preclinical testing by Cellics Therapeutics, which is currently in the process of submitting an IND application to the FDA for their lead red blood cell nanosponges for the treatment of methicillin-resistant staphylococcus aureus (MRSA) pneumonia. The company estimates the first patients in a clinical trial will be dosed next year. The UCSD researchers have also shown that nanosponges can deliver drugs to a wound site, sop up bacterial toxins that trigger sepsis, and intercept HIV before it can infect human T cells.
The basic construction for each of these nanosponges is the same: a biodegradable, FDA-approved polymer core is coated in a specific type of cell membrane, so that it might be disguised as a red blood cell, an immune T cell or a platelet cell. The cloaking keeps the immune system from spotting and attacking the particles as dangerous invaders. “I think of the cell membrane fragments as the active ingredients. This is a different way of looking at drug development,” said Zhang. “For COVID-19, I hope other teams come up with safe and effective therapies and vaccines as soon as possible. At the same time, we are working and planning as if the world is counting on us.”






