The presence and activity of lymphocytes within a tumor is critical for determining the potential clinical response to cancer immunotherapy, such as immune checkpoint blockade. Typically, tumors with poor T-cell-inflamed phenotypes, often referred to as “cold” tumors, are associated with a modest clinical response.
High baseline infiltration of effector T-cell lymphocytes is considered “hot,” and patients are predicted to respond more favorably to treatment.
However, patient-to-patient response and durability remains highly variable. Thus, we need further understanding of how the immune compartment changes in response to immunotherapies in individual patients. More specifically, there is a serious gap in available methods for studying lymphocyte infiltration, trafficking, and spatial heterogeneity induced by different cancer immunotherapies in individual patients.
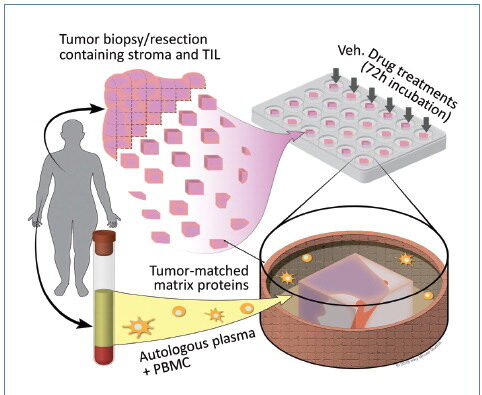
CANscript™ is an ex vivo human tumor model that recapitulates and preserves the native, patient-autologous tumor microenvironment, including autologous patient-derived peripheral blood mononuclear cells (PBMCs). The CANscript live-tumor assay reliably recreates the tumor ecosystem, including microenvironment components such as the stroma, the vasculature, and the immune contexture. In this system, therapies are tested by quantitatively assessing phenotypic response to therapy via a multidimensional set of assays, leading to a response-predictive score.
To train this platform, phenotypic response to treatment ex vivo was performed on ~2000 patients who received the same therapy in the clinic that was tested in CANscript. The results from the CANscript testing were correlated to patient clinical response using a machine learning algorithm, producing an “M-Score,” which predicts positive versus negative response.
To date, more than 4000 patient samples have been evaluated by CANscript. A subset of the clinical assay performance has been published, showing 90% clinical correlation.
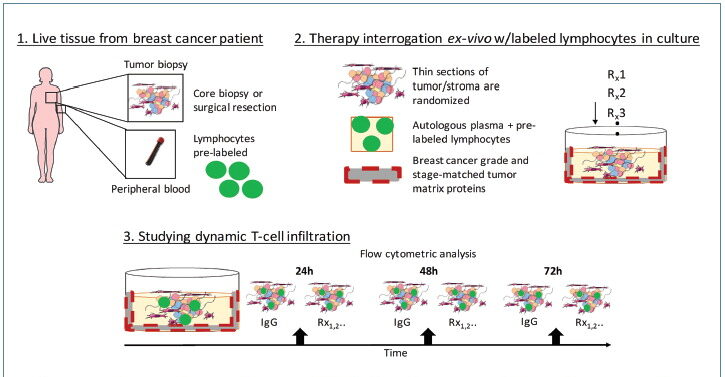
Preservation of the Tumor-Immune Contexture, Ex Vivo
In this example, utilizing tissue from head and neck squamous cell carcinoma and breast cancer patients, we characterized the tumor-immune contexture during the course of culture in the presence or absence of PD-1 checkpoint blockade.
To study the role of T-cell repertoires under different environmental and immunotherapy pressures, lymphocyte infiltration was characterized. Fluorescently labeled PBMCs were incorporated in CANscript and then studied using flow cytometric analysis.
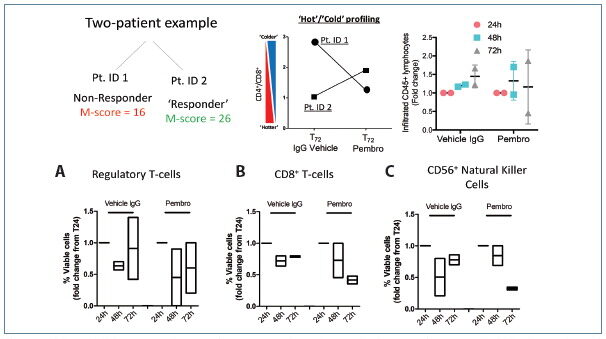
T-cell and immune subsets were quantified from vehicle and pembrolizumab-treated breast tumor CANscript, post-co-culture with PBMCs, at 24 h, 48 h, or 72 h to profile infiltration of Treg cells defined by CD4+CD25+CD127Lo, cytotoxic CD8+CD3+ T cells, and CD56+ Natural Killer cells.
Using technologies such as multi-spectral imaging and cytokine multiplexing, we can develop a more comprehensive understanding of the tumor microenvironment as lymphocytes dynamically traffic.
Capuring Nonuniform Infiltration
In this study, we determined that immune checkpoint blockade induced unique patterns of migration and infiltration of effector T cells (Teffs) and regulatory T cells (Tregs) in hot versus cold tumors. Furthermore, it was determined that, in some instances, cold tumors can be driven toward a hot phenotype characterized by trafficking of active immune lymphocytes following treatment, which corresponded to differential ratio of Teffs to Tregs compared to baseline.
The dynamic, spontaneous migration of tumor-infiltrating lymphocytes (TILs) is a key factor for immune-dependent tumor rejection. However, little is known about the role of immunotherapy or other anticancer agents as they modulate TILs. Not only were we able to capture the dynamism of the tumor microenvironment, the range of behavior in T-regulatory versus T-effector cells indicates patient-specific response to PD-1 blockade.
CANscript enables a platform to study the antitumor effect of different therapies along with stochastic lymphocyte infiltration at the individual patient level. By integrating an algorithm-driven predictive clinical assessment (M-Score), CANscript can contribute to a unique understanding of the role dynamic TILs play during the clinical response to therapy.
These data demonstrate that high interpatient variability in immune modulation, alterations to the tumor microenvironment under drug pressure, and predicted clinical response to immunomodulators requires a multidimensional approach to understand the effect of drugs on a per-patient basis.
Taken together, these data exhibit the utility of CANscript as a platform to characterize response to immunotherapy in a spatial context, providing insight into the migratory patterns of immune-cell subsets at the individual patient level.
Such an advance in our preclinical methods to study immunomodulators may help guide treatment decisions for clinicians while simultaneously functioning as a platform to study and discover mechanisms of clinical efficacy for emerging drug combinations.
Aaron Goldman, Ph.D. ([email protected]), is director, R&D, Immuno-Oncology, at Mitra Biotech.






