February 1, 2018 (Vol. 38, No. 3)
William Iannuccilli Contributor GEN
Microfluidic Readouts Could Flag Problems and Open the Door for New Treatment and Potential Early Intervention
Maze-like medical challenges are being navigated in miniature—in microfluidic devices. These chip-based networks interconnect channels and chambers as intricately as the original Labyrinth, but they are most impressive—and useful—when they are being used to capture biomarkers, screen drugs, monitor patients, or recapitulate physiological mechanisms.
If such tasks are to be completed, the users of microfluidic devices must avoid dead ends. Fortunately, these users—researchers, drug developers, and clinicians—can keep threading dark alleys if they stay current with the latest microfluidic technologies.
Microfluidic applications are being introduced that better reflect disease states in patients, opening doors for new treatment regimens and, potentially, early interventions. Also, microfluidic technologies can manage with small and safely acquired patient samples while consuming reagents in agreeably tiny volumes. Little wonder, then, that microfluidic technology is becoming more attractive to the medical community.
Opportunities for Noninvasive Testing
The laboratory of Shannon Stott, Ph.D, assistant professor of medicine at Harvard Medical School and assistant in genetics at Massachusetts General Hospital, uses microfluidic technologies to isolate circulating tumor cells (CTCs) and extracellular vesicles (EVs) from brain cancer patients. Dr. Stott anticipates that microfluidic approaches could complement traditional clinical approaches and derive more information from blood-based tests.
For brain cancer patients, surgical biopsy procedures pose such dire risks that they are performed sparingly, so sparingly, in fact, that biopsy tissue is a limited source of information about a tumor’s response to treatment. Brain tumors are typically monitored radiologically via magnetic resonance imaging or computed tomography, technologies that may produce misleading images. For example, these technologies may capture pseudoprogression, a post-treatment state that merely looks like tumor progression on early imaging.
Dr. Stott and her colleague Brian Nahed, M.D., a neurosurgeon at Massachusetts General Hospital, are participating in the development of microfluidic blood-based tests that have the potential to use CTC and EVs as surrogates for glioma biopsies. “Our approach,” says Dr. Stott, “enables very gentle but rapid processing of whole blood, such that we are able to isolate tumor products from blood with high sensitivity and specificity.” Drs. Stott and Nahed hope that the new approach yield patient blood signatures that will not only guide patient treatments more effectively, but also improve the referral processes that match patients with clinical trials.
The capture of CTCs may be accomplished with the CTC-iChip (Figure 1), a device that works by means of negative selection. That is, the CTC-iChip removes everything from a blood sample except for the rare cancer cells of interest.
The isolation of CTCs is often said to be a difficult as s finding a needle in a haystack because they are so rare. In blood samples, CTCs may account for no more than one cell in a billion.
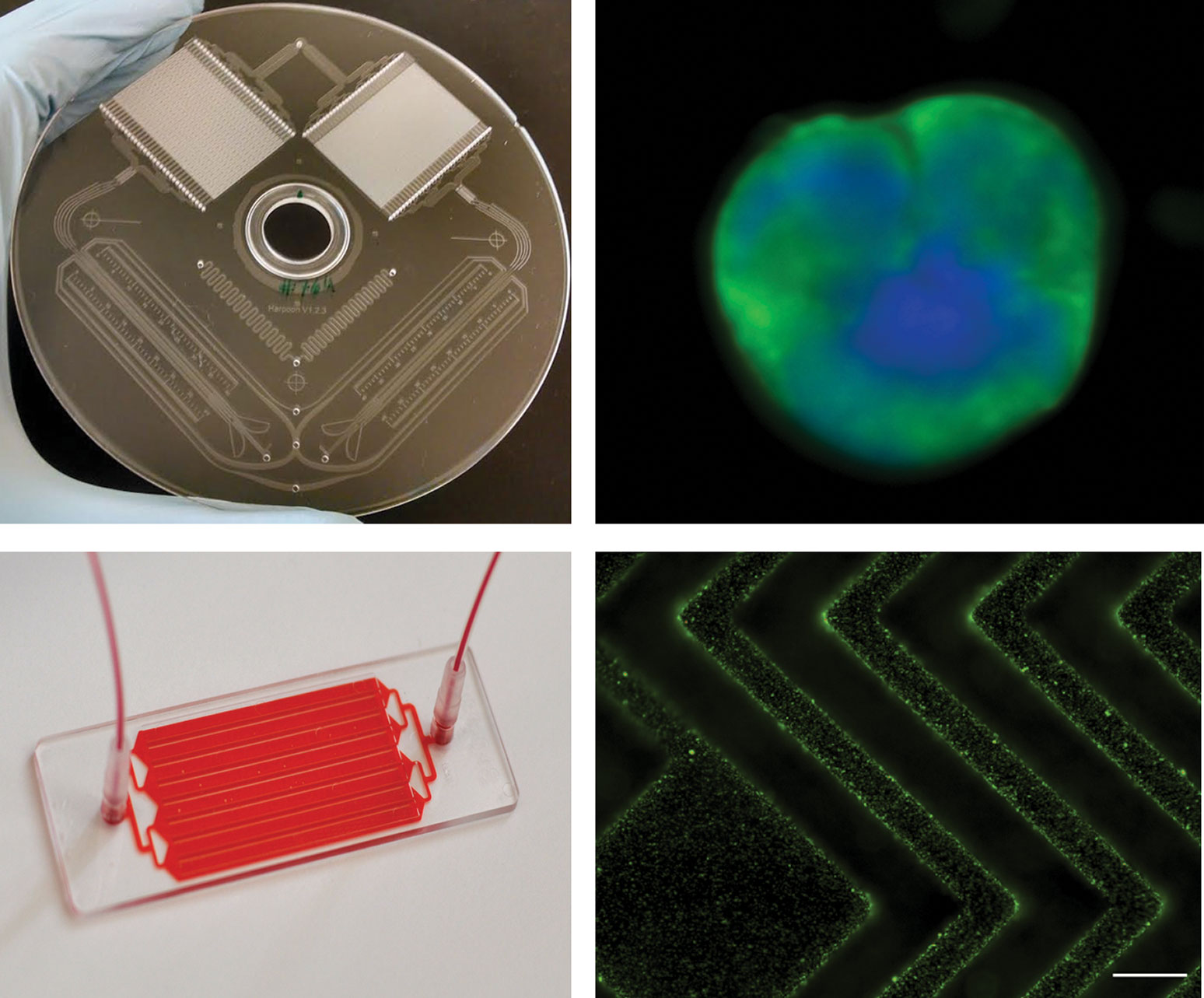
Figure 1. Scientists at Massachusetts General Hospital developed the CTI-iChip, which can isolate circulating tumor cells (CTCs), and the EVHB-Chip, which can isolate extracellular vesicles (EVs) released by cancer cells. Upper left: The CTC-iChip. Upper right: CTCs that came from a glioblastoma patient and were isolated with the CTC-iChip. Lower left: The EVHB-Chip. Lower right: EVs that came from a cancer patient and were isolated with the EVHB-Chip.
Using negative selection, however, researchers can remove the hay and leave the needles. Since the researchers know what the hay looks like (white blood cells, red blood cells, and platelets), they can target those for removal using microfluidic manipulations. “The cancer cells remain,” emphasizes Dr. Stott. “Importantly, they will be untouched and unlabeled, enabling robust downstream assays.”
EVs may be isolated with the EVHB-Chip (Figure 2), which uses a positive selection approach.” Almost every cell in the body releases EVs, but the EVs released by brain tumors can be selectively isolated on the basis of the proteins they carry, which include proteins found in brain tumors. The inner surfaces of the EVHB-Chip have been designed to promote maximum interactions between small EVs and the specific antibodies that target the brain proteins, allowing a 100-fold enrichment of tumor vesicles.
“For both technologies, we incorporate all processing steps ‘on chip,’ abstaining from harsh measures such as the centrifuging of blood samples or the lysing of red blood cells,” explains Dr. Stott. “This helps to maximize our biomarker yield while also providing high-quality RNA and even the possibility of growing the cancer cells in the lab.” The clinical relevance of this technology will allow tumor tracking, better stratification of treatment needs, and better matching of patients to clinical trials.
Dr. Stott hopes that in the future, researchers can expand applications to other neurological diseases (as well as traumatic brain injuries) and increase sample stability from the time of draw and down the processing line.
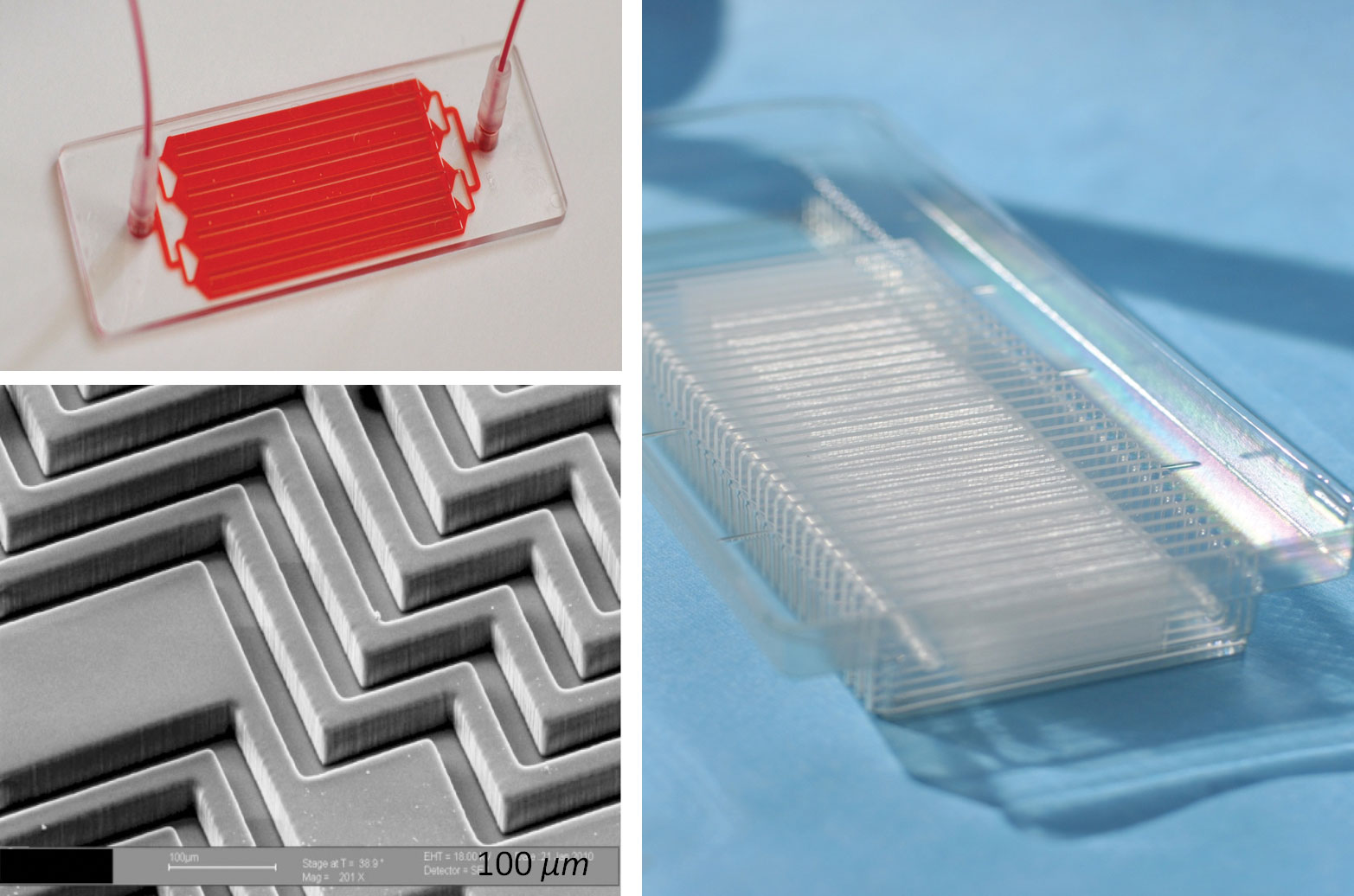
Figure 2. The EVHB-Chip, which was developed by scientists at Massachusetts General Hospital, uses the principle of positive selection to isolate extracellular vesicles shed by cancer cells. Upper Left: The EVHB-Chip. Lower Left: SEM image of the herringbone grooves on the surface of the EVHB-Chip. These grooves promote mixing of the blood and plasma, promoting interactions with the antibodies used to capture cancer biomarkers. Right: Scaled version of the EVHB-Chip, made in plastic for broad distribution.
Monitoring Health in Real Time
Abraham Lee, Ph.D., William J. Link professor and chair of the Department of Biomedical Engineering, University of California, Irvine, states that we are entering what he calls the golden age of microfluidics for medicine. He notes that in the past, we took samples from patients and received results in days and even weeks using in vitro diagnostics and analysis. But now, he adds, in vivo and in vitro technologies are merging so that real-time analysis is becoming possible.
Dr. Lee suggests that microfluidics can be used to monitor human health the way a car’s sensors are used to monitor how well the car is operating. Microfluidic readouts could, like a car’s dashboard indicators, flag problems as they arise and allow them to be resolved before they become worse.
If a car’s fluids can be monitored in real time, why not a human’s fluids, such as blood? Curiously, car maintenance is in some respects more technologically advanced than human maintenance, even though most would agree that the latter is much more important.
Microfluidics applications being developed than can perform real-time health analysis and reveal problems at their onset, when they may be more easily resolved. For example, several clinical applications are incorporating the “microfluidic circulatory system” concept. These include liquid biopsy, organ on a chip, single-cell analysis, and immunotherapy.
Dr. Lee’s group has developed an integrated microfluidic chip platform that uses acoustic streaming to separate all the major components of blood. Also, the platform can isolate blood cells—white blood cells, red blood cells, and rare cells such as CTCs—for single-cell analysis. The group has also developed microfluidic single-cell analysis techniques capable of facilitating genotypic/phenotypic, transcriptomic, and metabolomic analyses—all of which may serve diagnostic purposes. Acoustic streaming is generated by a vibrating interface that creates local mechanical motions that spread out and result in the formation of vortices, which enable the separation of different target cells.
Dr. Lee and his collaborators have developed an organ-on-a-chip technology that can recapitulate the patient’s microcirculatory system by growing a microphysiological system on a microfluidic chip. On the chip, mechanical and chemical stimulation is used to create tissue that has its own vascular network.
The technology allows “live” monitoring, which can be used to improve health assessments treatment plans. It is also being commercialized by Dr. Lee and his collaborators at 4Design Biosystems as a drug-screening platform. The company indicates that it has developed multiple physiologically relevant micro-organ systems that receive nutrients and drugs through living blood vessels, just as tissues do in the body.
Whether healthy or ill, a person could be monitored via the holy grail of modeling: a microfluidic chip that contains cells taken from that person’s own body. Such a chip could replicate an ill person’s unique disease state. A patient’s tumor, for example, could be faithfully modeled in miniature.
Future developments could incorporate wearable microfluidic sensors for monitoring health status in real time via a computer interface. The goal of individualized or personalized medicine in a microcirculatory diagnostic system appears to be within reach using this technology.
Advances Rooted in Drug Development
“Organ-Chips are microengineered systems that recreate human biology,” says Daniel Levner, Ph.D., chief technology officer at Emulate. “They permit the study of physiology and pathophysiology in an organ-specific context.”
Emulate makes Organ-Chips, microfluidic versions of organs such as lung, liver, intestine, and brain. Each Organ-Chip is composed of a clear flexible polymer about the size of a AA battery and contains hollow channels lined by living human cells (Figure 3). The cells are cultured under continuous flow and mechanical forces, thereby recreating key factors known to influence cell function in vivo.
“Cells cultured in Organ-Chip microenvironments go beyond conventional 2D and 3D in vitro models by recapitulating in vivo intercellular interactions, critical tissue architecture, spatiotemporal gradients, vascular perfusion, and mechanical forces—all of which are key for human relevant cell function and gene expression” notes Dr. Levner. Emulate’s Organ-Chip platform is designed to provide key insights into disease and drug mechanisms in a dynamic system that can be observed in real time.
Organ-on-a-chip technology was initially developed to address the needs of drug development. By some accounts, 90% of compounds that enter clinical trials fail, despite extensive testing using multiple in vitro and animal models. These conventional models, a growing body of evidence suggests, may be outperformed by microfluidic models incorporating organ-on-a-chip technology. Besides being more predictive of human safety and efficacy than existing cell culture and animal models, organ-on-a-chip models may provide a unique window into biological mechanisms.
This possibility can be explored with Emulate’s Human Emulation System, which integrates Organ-Chips, instrumentation, and software. As part of the Human Emulation System, Organ-Chips may do more than show whether a compound is safe or efficacious. It can also show how a compound works, or doesn’t. According to Emulate, the Human Emulation System can provide a high-fidelity window into the inner workings of human biology. This technology, the company indicates, is already being used in the food and cosmetics industries, in regulatory and government bodies, and in basic disease research.
Emulate is working to democratize its technology, turning it into a lab-ready commercial platform that can be used by any researcher with cell-culture experience. When discussing about Emulate’s future, Dr. Levner emphasizes two key application areas: improving drug development and creating patient-on-a-chip devices. “By combining our Organ-Chips with induced pluripotent stem cells,” says Dr. Levner, “we have the potential to open a new world of possibilities for precision medicine.”
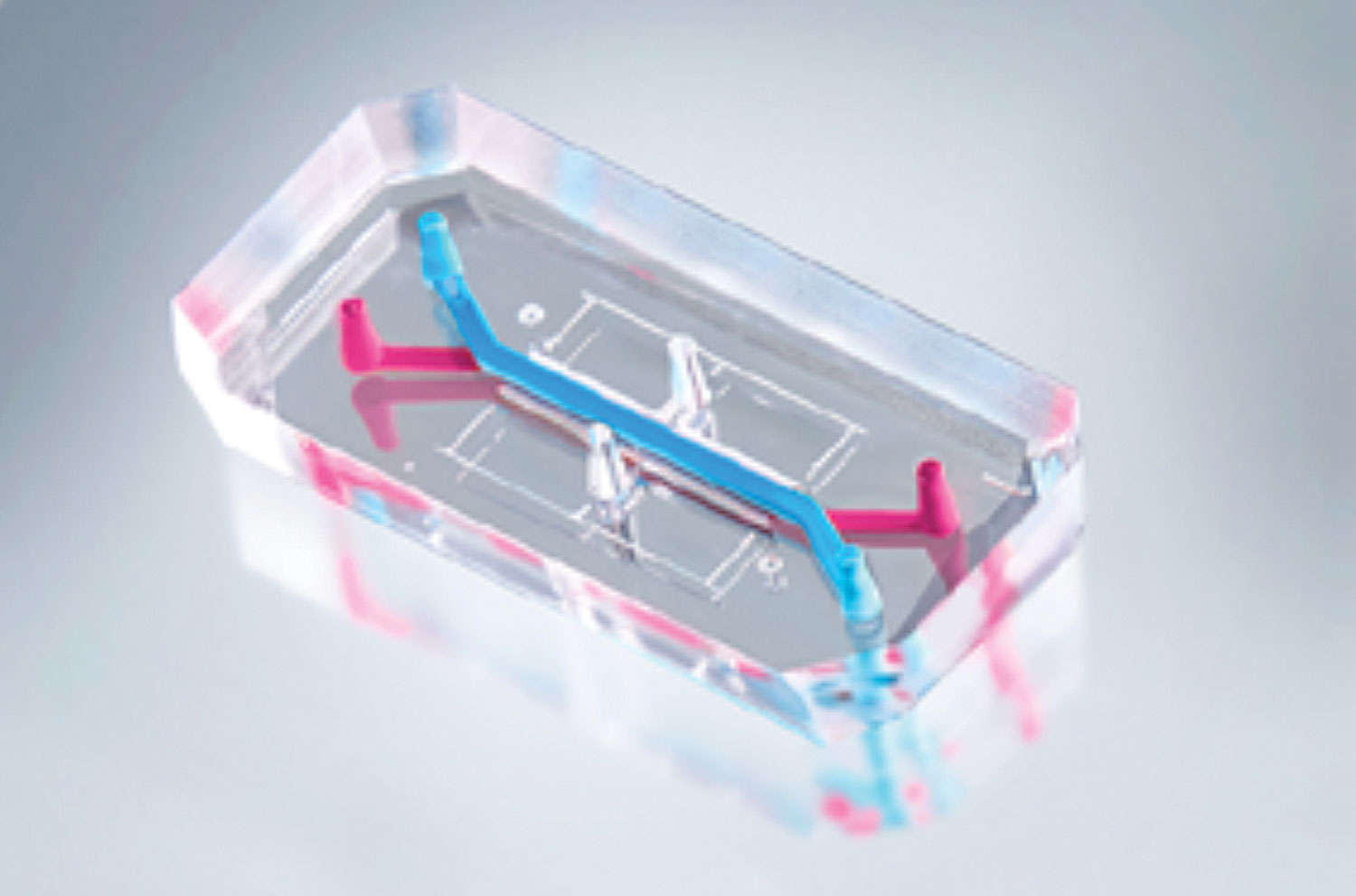
Figure 3. Emulate develops Organ-Chips, living products for understanding how diseases, medicines, chemicals, and foods affect human health. The Organ-Chip is designed to recreate aspects of human physiology, such as the extracellular matrix, tissue-tissue interfaces, mechanical forces, biochemical surroundings, and immune system components.







