August 1, 2014 (Vol. 34, No. 14)
H. Tschiersch Researcher Leibniz Institute of Plant Genetics
G. Liebsch PreSens
L. Borisjuk Researcher Leibniz Institute of Plant Genetics
A. Stangelmayer CEO PreSens
H. Rolletschek Researcher Leibniz Institute of Plant Genetics
Metabolic Activity of E. coli Monitored
Inside the Incubator with the VisiSens™ A1
Microbial and cell/tissue cultivations play major roles and serve as important tools in biological and medical research. Knowledge about the local metabolic activity of cells and structures within colonies may assist in understanding and controlling complex growth processes.
One key parameter in cultivation control is oxygen. Oxygen consumption or production can give valuable information about the metabolic status of a culture. Thus, precise oxygen measurement systems are indispensable.
However, conventional techniques, e.g., electrodes, only allow single-point measurements, lacking the spatial information about oxygen distribution in the sample. Even conducting transect measurements with oxygen microsensors cannot completely overcome this limitation.
The VisiSens™ A1 system combines planar fluorescent sensor foils with digital camera technology, and now allows two-dimensional assessment of oxygen distribution across the sample surfaces. The fluorescent sensor foils are non-toxic and do not consume oxygen during the measurement process.
Information over a whole area can be recorded with μm resolution for extended measurement periods. The sensor foils are read out, contactless with a compact fluorescence microscope. It can be easily installed inside an incubator and is controlled from the outside by PC or notebook (Figure 1).
Being able to visualize and evaluate changes in oxygen partial pressure due to metabolic and diffusion processes makes VisiSens an applicable tool for cell and tissue culture monitoring. We tested this measurement technology and investigated oxygen distribution in different plant tissues. We also tried it in a nonplant application.
In the experiment described here E. coli was used as a model microorganism. The culture was monitored with the VisiSens A1 system inside the incubator, and the oxygen consumption of single bacteria colonies was visualized.
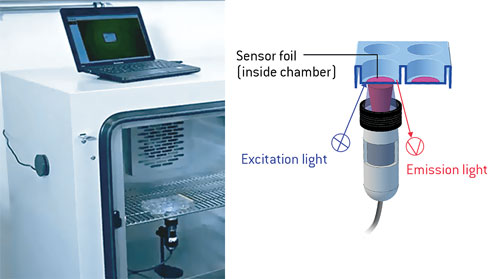
Figure 1. VisiSens A1 installed inside an incubator for noninvasive oxygen monitoring in a multiwell plate. The device is controlled from outside via notebook.
Materials & Methods
At the start and the end of the experiment the VisiSens oxygen sensor foil (SF-RPSu4, PreSens) was calibrated by recording images of both a sodium sulfite solution (0% air saturation) and of air-saturated distilled water (100% air saturation). E. coli colonies were grown on an agar plate, and the sensor foil was put directly on the culture.
The VisiSens detector unit (DU01) was installed inside the incubator and controlled from the outside with a laptop. Detector unit control and subsequent image analysis were realized with VisiSens AnalytiCal 1 software. Image recording was started immediately after the culture was put in the incubator for an incubation period of 20 minutes; images were taken at an interval of 30 seconds.
Imaging of E. coli Colonies
Short-term incubation (5–20 minutes) with the oxygen sensor resulted in clear oxygen distribution images, where single colonies were identifiable by their oxygen consumption (Figure 2).
The oxygen concentration at the location of the colonies decreased rapidly within 20 min (Figure 3). The oxygen images further reveal how the oxygen decrease depends on the distance to other colonies. Driven by diffusion and therefore by oxygen consumption of multiple neighboring E. coli colonies the oxygen level inside the medium also changes over time.

Figure 2. Oxygen image of E. coli colonies growing on agar. Yellow color indicates high oxygen; blue color, low oxygen concentrations.
Conclusion
VisiSens is able to visualize different sites within a multiwell plate or Petri dish. Applied to microbial culture it is possible to distinguish oxygen consumption of single colonies. By visualizing oxygen distributions across the sample and its changes over time valuable information can be gained, which can be used to monitor the metabolic status or to modulate oxygen supply to better control certain processes in cell or tissue culture.
The use of VisiSens facilitates the study of respiratory dynamics and will contribute to a better understanding in many application fields, like the screening for respiratory phenotypes in yeast.

Figure 3: Time series recordings of E. coli colonies during short-term incubation. Oxygen distribution changes and consumption over time can be clearly determined. Yellow color indicates high oxygen; blue color, low oxygen concentrations.
H. Tschiersch, L. Borisjuk, and H. Rolletschek are researchers in the dept. of molecular genetics, Leibniz Institute of Plant Genetics. G. Liebsch ([email protected]) is head of R&D imaging solutions and A. Stangelmayer is CEO at PreSens.







