August 1, 2011 (Vol. 31, No. 14)
Vicki Glaser Writer GEN
Choosing between Stainless Steel and Single-Use Getting Easier as Niches Become Better Defined
Even as single-use process technology continues to advance and evolve to meet a growing demand, there remains a vibrant market for clean-in-place systems ranging from benchtop autoclavable vessels to stainless steel tanks for applications ranging from microbial fermentation to scale-up and large-scale cell culture.
From bags mounted on rocking platforms for mixing, to the introduction of stirred-tank single-use vessels and other innovative mixing strategies designed for improving the efficiency of aeration, feeding, and waste removal to boost cell densities and product yield, single-use systems continue to evolve.
Design modifications have facilitated larger processing volumes, simplified transport and manipulation of cell bags, and enabled greater online process monitoring and automated process control by introducing multiple ports and single-use sensors.
“Today we finally understand the benefits of each technology,” stainless steel and single-use, “and that none is perfect,” says Doru Felezeu, director of marketing and business development at Pierre Guerin Technologies. While single-use offers advantages in time, labor, energy, and validation with the elimination of cleaning and sterilization of the vessel, it introduces concerns related to security, waste, capacity, reliability, and automatability, he adds.
Despite overall growth in the single-use bioreactor market, demand for these systems is still project-, market-, and customer-specific, as well as regionally driven, with single-use technology not yet widely adopted in some areas, according to Richard Mirro, product manager at New Brunswick Scientific.This may be attributable to regional differences in energy, water, and labor costs. Uptake may also vary depending on an organization’s need to transfer processes between sites and the requirements for flexibility and rapid turnaround in a multiproduct facility.
GE Healthcare Life Sciences, makers of the Wave platform of single-use bioreactors, has approached the debate over whether stainless steel or single-use technology is more advantageous at various production scales by conducting a study and generating data. It has concluded that “there is a limit to [the advantages of] single-use technology, and it is contingent on two main factors: kg/yr requirement and, most importantly, facility utilization,” says Richard Ferraro, senior product manager.
GE evaluated a mAb production process in a facility that produces 10 products/ year, for a total production of 800 kg/ yr. The company compared two process models: five 2,000 L stainless steel tanks or 20 500 L disposable bioreactors. GE used modeling, scheduling, and facility design software, and the results demonstrated that single-use technology was a better solution at the given scale.
Although the ultimate output with single-use reactors fell short of the 800 kg goal, it was 34% higher than the amount produced in steel tanks, reports Ferraro, mainly due to faster turnaround time (without the need for cleaning/sterilization). Other benefits included an 18% head-count reduction and a decrease in capital investment of about 40%.
The above results held true, however, if facility usage was above 90%. When facility usage dropped below 90%, “stainless steel was the way to go,” which Ferraro attributes to the cost of the single-use technology.
“We found that the single-use sweet spot has to do with utilization,” he says. It is ideal for facilities that require about 100 kg/yr of a product that is produced in 500 L–1,000 L working volume reactors, such as for pilot-scale production or cGMP manufacturing of clinical trial material, and for facilities that manufacture multiple products a year and need maximal flexibility, such as a CMO.
In response to increasing demand for single-use bioreactors for use in smallscale cell culture applications, Sartorius Stedim Biotech recently introduced the UniVessel® SU bioreactor with a 2 L working volume. The reactor is presterilized and is compatible with all of Sartorius Stedim Biotech’s Biostat® benchtop bioreactor controllers, as well as other controllers designed for conventional glass reactors.
Top plate ports can accommodate pH and DO sensors and Pt100 temperature probes. Sartorius designed the SU vessel to have a torospherical bottom and a height-to-diameter ratio similar to the company’s glass UniVessel.
Additions to the company’s Biostat STR line—stirred-tank single-use vessels available in 50 L and 200 L volumes—will include 500 L and 1,000 L vessels designed to model stainless steel reactors to facilitate scale-up. They will launch toward the end of 2011. During the third quarter of this year, Sartorius Stedim Biotech plans to introduce the Biostat ORB that uses an orbital shaking motion for mixing and is intended for scale-up from shake flasks, for example for inoculum production or as an alternative option for protein production. The first product in this line will be a 200 L single-use bioreactor.
Christel Fenge, Ph.D., vp product development and marketing fermentation at Sartorius Stedim Biotech, agrees that the choice between an autoclavable/clean-in-place system versus a single-use unit remains largely application dependent. Even for microbial fermentation, traditionally done in stainless steel tanks, she reports rising interest in the company’s 50 L single-use system. It may be easier to use a disposable reactor bag for certain applications, such as when working with toxic products or certain strains of E. coli, for example.
Overall, fermentation represents a “stable business model,” says Richard Mirro of New Brunswick Scientific, which introduced the BioFlo 610 sterilize-in-place pilot- scale reactor to capitalize on the growth in fermentation applications.
Concurrent with single-use systems becoming widely accepted in the industry, there is continuing market pressure to improve their performance and productivity and to fill in gaps, such as in the availability of single-use sensors and benchtop units, according to Ken Clapp, senior director, global marketing and product management at Xcellerex.
Based on the company’s experience operating a biologics manufacturing facility, designing equipment, and integrating a variety of bioprocessing products, “The industry still has a way to go to achieve a totally disposable bioprocess factory,” Clapp adds.
With regard to single-use sensors, customers are looking for improved range, repeatability, accuracy, and robustness, as well as broader variety. They want monitoring capabilities beyond pH and dissolved oxygen, reports Clapp, such as sensors for measuring biomass and carbon dioxide.
Demand is also increasing for single-use systems that can replace benchtop stirredtank, glass bioreactors that better represent larger-scale bioreactor systems at volumes of 50 L and higher.
“Tremendous gains have been made at the larger sizes. Now end-users want the same at the bench,” says Clapp. “Customers don’t have time to work through the technology gaps between conventional systems and single-use systems. Having a benchtop system that is the same as the larger systems will save time and reduce risk and wasted efforts—and get biologics to market faster.”
Bag integrity is an ongoing area of concern among users. “We have heard of alarmingly high failure rates in bioreactor bags with some suppliers,” he adds.
Xcellerex recently introduced the XDR-10 benchtop bioreactor, expanding its product range from 10 L to 2,000 L for enhanced linear scalability. The new system includes an agitation mechanism, vessel geometries, bag bioreactor, polymer composition, automation system (Rockwell/ WonderWare software), and other features consistent with those used in pilot- and production-scale bioreactors.
The XDR-10 uses a stand-alone controller with a touch-screen interface and vessel stand and is available with single-use sensors for pH and dissolved oxygen. The models differ primarily in the number of mass flow controllers and peristaltic pumps.
The demand described by Clapp for small-scale, single-use bioreactors that are as similar as possible to larger scale glass and stainless steel vessels, can be used for cell-line selection and process-development studies, and enable reliable and predictive scale-up and transfer of processes echoes across the industry.
For example, during the third and fourth quarters of this year, New Brunswick Scientific will expand the range of its single-use portfolio—adding to its 5 L and 14 L vessels— with the addition of larger vessel sizes, accessories, and unique configurations. A collaborative development program with Pall will result in a new product launch toward the end of 2011 or in early 2012. The joint effort merges Pall’s Allegro™ single-use biocontainer platform and New Brunswick Scientific’s CelliGen® bioprocess controller.
Bioengineering recently introduced the PEEKRalf fermentor for growing extremophiles—organisms that thrive in extreme conditions such as high salt concentrations, high temperatures, or extreme pH values. These conditions would corrode the stainless steel components of conventional fermentors, explains Valentin Splett, subsidiary director.
“We have replaced all stainless steel parts in the autoclavable laboratory fermentpr Ralf with PEEK components,” which can withstand acidic, alkaline, and salt-containing solutions, even at high temperatures, Splett says. “Because of its modular build, any standard Ralf system can be transformed into a PEEKRalf.” Later this year Bioengineering will launch the Ralf- NOVA autoclavable fermentor, featuring a redesign of the company’s Ralf product.
Driving developments on the stainless steel side of the business is growing demand for standardized versus customized bioreactor solutions, according to Fenge. To meet this need, Sartorius Stedim Biotech is preparing to introduce 30 L and 200 L versions of the new stainless steel stirred tank bioreactor Biostat D-DCU. Standardization and the availability of prefabricated components will “improve reliability and decrease delivery timelines and price pressures.” The modules can be configured to create a system that meets customer needs.

Sartorius Stedim Biotech’s UniVessel® Culture Vessel, which is available in 0.5 L, 1 L, 2 L, 5 L, and 10 L working volumes, was designed to fit all of the company’s autoclavable fermentors and bioreactors.
Optimizing Process Development
What was an emerging trend five to ten years ago is now an accepted fact: by doing better quality experiments upstream of your bioreactor you can develop higher-titer and better quality products and processes,” says David Laidlaw, small-scale technologies manager at Applikon. Users want to run more experiments in the same bench space to gain a better understanding of their processes, and to perform cell-line screening and selection at higher throughputs and smaller scales, explains Laidlaw. By “upstream” he means before moving into a 3 L stirred tank vessel, where much process-development work has traditionally been done.
Applikon recently introduced the first version of its new miniBio small-scale stirred tank reactors, with a 250 mL vessel capable of supporting 50 mL to 200 mL working volumes. Subsequent vessels will be available in 500 mL, 1,000 mL, and 3 L volumes. Introduced along with the miniBio is Applikon’s new my-Control ™ controller that offers the same capabilities as the Applikon EZControl unit in a smaller footprint. “Five complete systems—vessels and controllers—can now fit in one meter of bench space,” notes Laidlaw.
Also new from Applikon for early-stage cell culture work including cell-line creation and screening is a 24-well deep-well plate. Made of ultrapure polystyrene, this new cassette is gamma-irradiated, features a pyramid-shaped bottom, and can be used with the uFlask well plate closures in place of shake flasks.
Companies continue to embrace the principles of process analytical technology (PAT) and incorporate them into their processoptimization strategies. The latest version of Sartorius Stedim Biotech’s multifermentor control software, for example, offers design of experiments (DoE) and multivariant analytical tools to support PAT.
Ferraro of GE reports that the company’s new WavePOD II control module is the result of two years of collaboration with clients to understand the relative sensitivity of various process parameters and “to make PAT more realistic for the end-user” and more of a plug-and-play operation. The controller integrates all of the readouts from the Wave Bioreactor 20/50 system, including DO, optical pH, and CO2/O2 gas mixing controls and automatically varies the rocker speed or oxygen concentration supplied to the Cellbag to maintain critical process parameters within the desired ranges.
The ability of a control system to monitor process parameters in real-time and to make changes in response to those measurements is particularly critical for perfusion processes, a growth area in the bioprocess market, according to Ferraro. With the continuous addition of media and extraction of product over periods as long as weeks, cell density can reach a high level and the system may exchange thousands of liters of media. Perfusion systems are very sensitive to small changes and “can become very unstable.”
Single-use sensors are another evolving area. Ferraro describes all of GE’s sensors as single-use: they either have direct product contact and are disposable, or they are inserted into a cell bag through a sleeve or sheath, do not directly contact the product, and can be re-used without cleaning. The company is moving toward adding more embedded sensing capability into its bags to meet the demand for monitoring processes such as cell density, glucose, and other types of metabolic parameters.
The main challenge, Ferraro explains, is fabricating single-use sensors capable of making these measurements that can withstand the gamma irradiation used to sterilize the bags.
Focusing on the single-use cell bag, GE plans a redesign of its Wave bag by the end of this year. The new line of standard cell bags will incorporate in-demand features of its customized versions, including more connections and greater use of aseptic connectors. One goal of the new bag design will be to accommodate more intensive cell culture processes such as perfusion cultures and to enable increased monitoring of acid/base additions and feeding and harvesting of the cultures, for example.
Additionally, companies such as GE are taking a close look at the film/plastic used to manufacture the disposable cell bags, as the FDA and their customers are becoming increasingly concerned about the effects of extractables and leachables.
Innovating Process Streams
Market demand for parallel unit operations for process design and optimization studies was the underlying principle for the design of HexaScreen’s automated multiple minibioreactors system, sold under the brand name Telstar HexaScreen.
The system supports animal cell culture on a scale of 10 mL to 15 mL. With the HexaBatch model, users can perform six experiments in parallel in single-use interchangeable plates, with online monitoring of temperature, DO, and pH.
Earlier this year Infors adapted its Labfors 4 Cell bioreactor system to be compatible with Millipore’s single-use Mobius™ CellReady 3L Bioreactor vessel, and a single XDDC control unit can operate four parallel bioreactors.
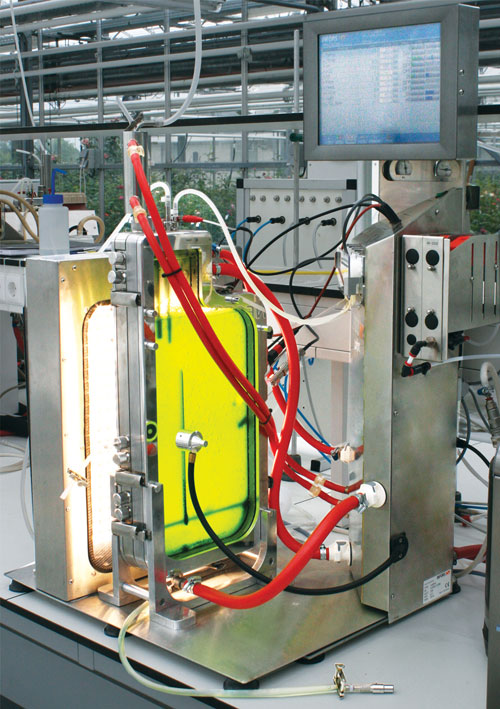
A collaboration between Infors Benelux and Wageningen University led to the design for a flat panel bioreactor for growing phototrophic microalgae and cyanobacteria, which is now in use at the Algae Production and Research Centre in Wageningen. Commercial versions of the photo-bioreactor are being produced by Infors in Switzerland and Appropriate Technical Resources (ATR) in the U.S.
The 2 L (total volume) autoclavable vessel connects to a standard Labfors console. The flat panel design of the photo-bioreactor supports high biomass densities. The system can monitor and control temperature, pH, DO, antifoam, and feed parameters, and is also capable of off-gas measurements.
ATR will also produce and market the CELLutions Biotech CELL-tainer® single-use bioreactor in North America. The two-dimensional mixing movement of the CELL-tainer supports high cell growth rates and allows the system to support both cell culture and microbiall fermentation processes, according to Steve Mitchell, CEO of ATR. It can accommodate 500 mL to 15 L working volumes and includes embedded pH and DO probes.
Felezeu of Pierre Guerin described the three largest markets for his company’s bioreactors and fermentors over the past year as vaccine manufacturing (from seed culture at 5 to 10 L volumes up to 1,000 L production scale), enzymes (largely for use in biofuels production), and a center in Ireland called the National Institute for Bioprocessing Research & Training, where people receive hands-on training to work in cGMP manufacturing facilities.
Felezeu also reports on the company’s ongoing development of software modules to enable more complete automation of bioprocesses. With mergers and acquisitions continuing in the pharmaceutical sector, creating extra bioprocessing capacity, Felezeu describes a healthy market for refurbishing, updating, and optimizing existing facilities, equipment, systems designs, and software to meet the needs of new users and new applications and to be in accordance with the changing regulatory landscape.
ATMI reports evidence of a deliberate focus on process intensification driven by the need for faster, less expensive, and more efficient cell culture technologies. With the acquisition of Artelis, ATMI has expanded its integrity bioreactor platform to include the iCellis perfusion-based reactor, which uses an inert substrate to immobilize attachment-dependent and suspension cells.
The Xpansion reactor from Artelis utilizes stacked disks to create a high density perfusion environment for growing stem cells and other cell therapy products. The PadReactor Mini is a benchtop and scalable version of ATMI’s PadReactor, which combines a square reactor vessel and the gentle PadMixer that incorporates sparging technology. Earlier this year, ATMI entered an agreement with Finesse Solutions to provide additional plug-and-play controller options that readily integrate with the Integrity PadReactor.
A collaboration between HexaScreen and Appropriate Technical Resources led to the development of a flat panel bioreactor for growing phototrophic microalgae and cyanobacteria.



