April 1, 2017 (Vol. 37, No. 7)
Gail Dutton
RXi’s Modiefied RNAi Molecules Slide into Immune Cells, Silence Immunosuppressive Genes
In January 2017, RXi Pharmaceuticals acquired MirImmune and launched itself into the immuno-oncology space. Previously, RXi had explored dermatology and opthalmology. As far-flung as these destinations may seem, they are all within reach of RXi’s self-delivering RNA interference (RNAi) technology, which is called sd-rxRNA®. At present, several sd-rxRNA molecules are in clinical trials. Thus far, all are for dermatological and opthalmological indications. But RXi is also moving quickly to extend its RNAi knowhow to cell-based cancer immunotherapies.
The sd-rxRNA molecules are designed for efficient cellular uptake. Although they are, in principle, amenable for systemic delivery, sd-rxRNA molecules have been deployed where local administration and high sd-rxRNA concentrations can be achieved. Hence the eye and skin applications. Like eye and skin cells, ex vivo immune cells are readily accessible. For example, T cells are isolated from patients to facilitate processing for adoptive cell transfer, a promising form of immunotherapy. The adoptive cell transfer approach developed by MirImmune silences genes that express immunosuppressive receptors on T cells. It is a form of checkpoint inhibition.
Back in 2015, MirImmune decided to advance its immunotherapy approach by arranging to use RXi technology. “We licensed our self-delivering RNAi technology to them in exchange for a percentage of equity in their company,” recalls Geert Cauwenbergh, Dr.Med.Sci., RXi’s president and CEO. “After 18 months, MirImmune had fabulous results that showed we have the potential to access targets with RNAi that are impossible to access with antibodies.”
This project demonstrated the value of RXi’s technology for cell therapy. It also highlighted synergies between the two companies and led, eventually, to RXi’s acquisition of MirImmune.
By combining RXi’s proprietary RNAi delivery technology with MirImmune’s immune checkpoint-inhibition and cell-based therapeutics, the acquisition created “a major value inflection point,” asserts Dr. Cauwenbergh. “Data obtained in animal models of cancer should put us on the radar of companies interested in oncology.
“The addition of immuno-oncology isn’t a shift in focus,” he insists. RXi will continue its work in dermatology and ophthalmology while embracing immuno-oncology, adding programs and collaborations to make the most of its opportunities.
“Treating cells with sd-rxRNA ex vivo to modify them by silencing checkpoint target genes could improve their therapeutic potential for oncology,” notes Dr. Cauwenbergh. At present, a key goal of RXi’s immuno-oncology program is to develop improved, cell-based therapeutics for hematologic and solid cancers.
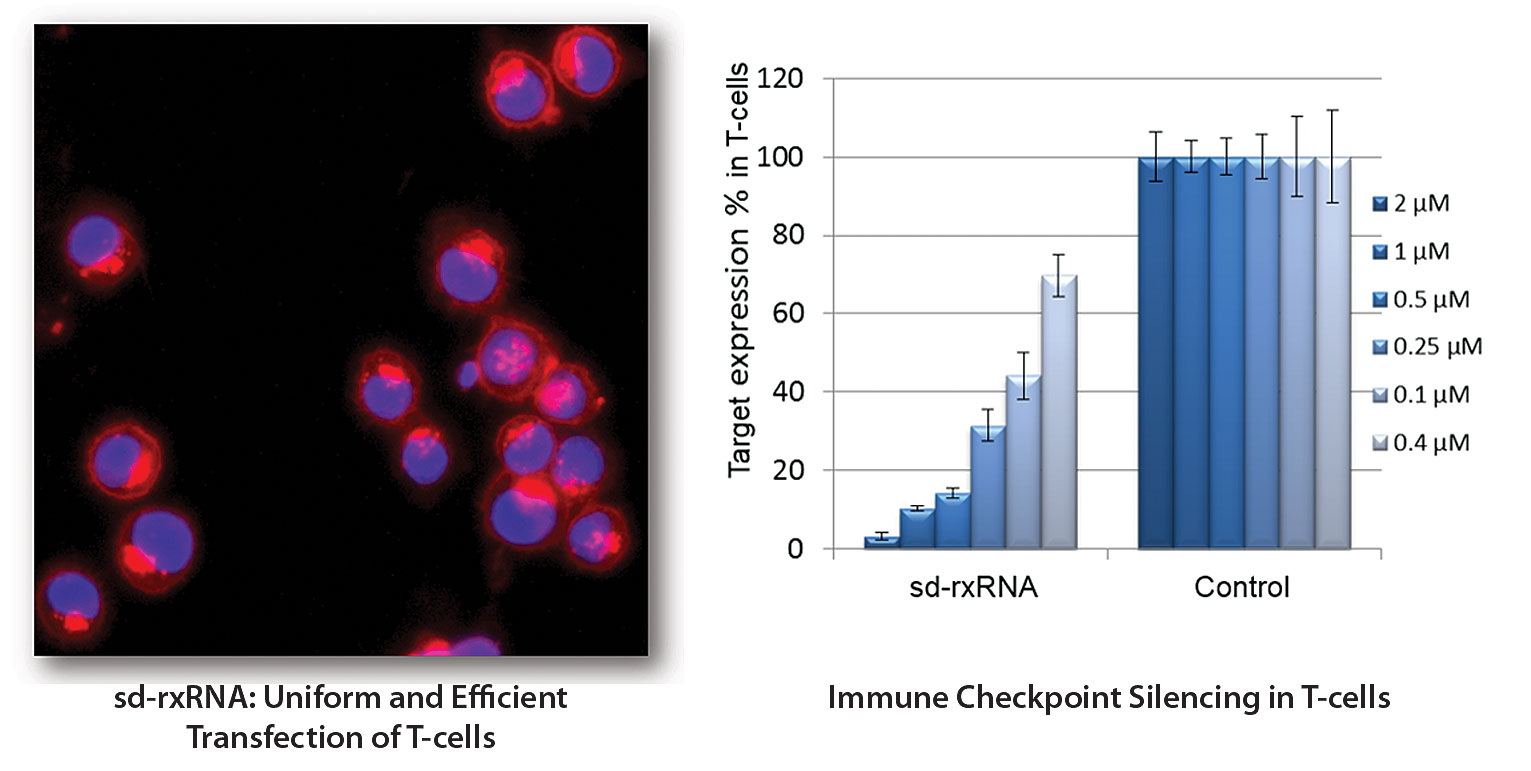
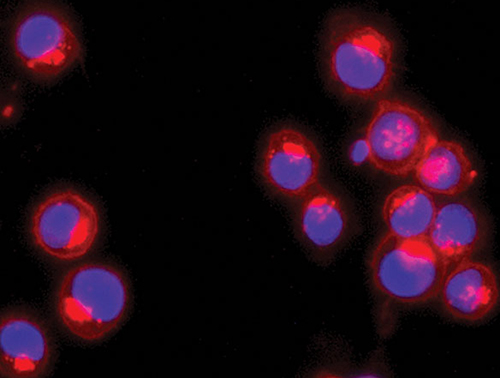
RXi Pharmaceuticals enhances the antitumor activity of ex vivo–propagated immune cells by treating them with self-delivering RNA interference (RNAi) molecules. Stabilizing and hydrophobic modifications enable these molecules to penetrate cells without any additional formulation or transfection procedure. The images here depict RNAi delivery (left) and gene knockdown (right) in human primary T cells.
Self-Delivering RNA
RNAi compounds have the potential to silence any human gene, including those that are otherwise undruggable. To achieve this potential, however, RNAi compounds need to acquire improved drug-like properties—potency, stability, and specificity. One way to obtain such properties is through chemical modification.
RNAi compounds may be modified with RXi’s sd-rxRNA platform, which incorporates RNAi and antisense technologies to create hybrid oligonucleotide compounds. These compounds have been shown to possess enhanced drug-like properties such as spontaneous cellular uptake, serum stability, reduced potential for immune stimulation, and long-lasting intracellular activity.
When used in the processing of immune cells ex vivo, sd-rxRNA compounds undergo efficient transfection and cause virtually no loss of cell viability. Additional work with immune cells originally undertaken by MirImmune has also demonstrated that sc-rxRNA technology may be used to silence multiple immune checkpoints. Even better, this form of checkpoint inhibition appears to lack the checkpoint toxicity that bedevils antibody-based approaches.
“We’ve shown, in mouse models, that anti-PD-1 sd-rxRNA-treated CAR T cells maintain reduction of the PD-1 checkpoint for at least one month after administration,” Dr. Cauwenbergh reports. “We are working to extend this duration, and believe that it will be competitive with PD-1 antibodies.”
Immuno-Oncology Has Challenges
The relatively limited body of research for immuno-oncology is one of the greatest challenges in this field. “We’re building the plane as we’re flying it,” he says. “But, in oncology, the need for effective therapeutics is urgent.”
Dr. Cauwenbergh expects this therapy to offer advantages other emerging technologies can’t provide. Immunotherapies offer revolutionary improvements in cancer treatment, but they have challenges that must be addressed. For example, Dr. Cauwenbergh points out, chimeric antigen receptor T cells currently work well for hematological cancers, but are not all that effective for solid tumors.
“Clinically used antibody-based checkpoint inhibitors have been highly effective in melanoma and in a rapidly expanding range of other cancers,” he adds. “However, antibodies target only extracellular checkpoints. Furthermore, the combinations of different types of biologics, while highly effective, are challenging in terms of toxicity as well as pharmacoeconomics.”
Dr. Cauwenbergh emphasizes that unlike antibodies, which block receptors that populate cell surfaces, sd-rxRNA molecules work inside the cell to prevent the receptors from ever sprouting. “The use of sd-rxRNA,” he declares, “may provide a unique solution for the modulation of multiple checkpoints in a single therapeutic treatment without major side-effects.”
RXi’s Business Opportunities
RXi realized that if it wanted to become a player in immuno-oncology, a competitive, fast-moving therapeutic area, the company would have to get up to speed. It decided that a good way to do so would be to enter a collaboration with MirImmune.
“The subsequent merger strengthens RXi’s existing clinical pipeline and provides access to a large immuno-oncology network of researchers to help move research in this new area forward,” comments Dr. Cauwenbergh. “We are planning work with a number of collaborative partners—both academic and industrial—to evaluate this technology in several different areas.”
Expansions of sd-rxRNA into dermatology and ophthalmology are probable. RXi’s lead clinical sd-rxRNA compound, RXI-109, is in a Phase IIa trial in dermatology and a Phase I/II trial in ophthalmology to reduce scar formation in the skin and eye, respectively. These two therapeutic areas, and others where local delivery is applicable, are potential areas of expansion through research collaborations, partnering, and/or licensing opportunities.
In addition to sd-rxRNA, RXi also has exclusive worldwide rights to Samcyprone™, a small molecule immunomodulator that triggers a T-cell response. Samcyprone is a topical formulation of diphenylcyclopropenone (DPCP) that can be used to treat warts, skin cancer, and alopecia areata (autoimmune hair loss). The FDA granted it orphan drug status to treat the cutaneous metastases for malignant melanoma patients in stages IIb to IV of that disease.
RXi is a small company, and developing RNAi therapeutics for three disease areas will be demanding, Dr. Cauwenbergh admits. But he has the advantage of collaborating with some of the world’s leading scientists and institutions. For instance, Craig Mello, Ph.D., co-recipient of the 2006 Nobel Prize in Medicine for the discovery of RNAi, is one of the company’s founders. RXi also has collaborations with scientists at numerous leading academic institutions and in industry.
RXi Pharmaceuticals
Location: 257 Simarano Drive, Suite 101, Marlborough, MA 01752
Phone: (508) 767-3861
Website: www.rxipharma.com
Principal: Geert Cauwenbergh, Dr.Med.Sci., President and CEO
Number of Employees: 15
Focus: RXi Pharmaceuticals is a clinical-stage company developing self-delivering RNAi therapeutics for immunotherapy, dermatology, and ophthalmology.