Glycosylation is one of the most abundant of all protein post-translational modifications (PTMs). It results from the addition of sugar residues to protein sidechains to form a glycoprotein. Mammalian glycoprotein oligosaccharides are commonly built from a limited number of monosaccharides but their structural diversity is vast, mainly because they often form complex branching patterns. Glycosylation is not template-driven and is currently impossible to predict.
Glycosylation plays an important role in many specific biological functions, including immune defense, fertilization, viral replication, parasitic infection, cell growth, inflammation, and cell-cell adhesion. For pharmaceutical glycoproteins, glycosylation affects stability of protein conformation, clearance rate, protection from proteolysis, and improves protein solubility.
A protein´ pattern of glycosylation depends on many factors, including the type of cell producing the glycoprotein, nutrient concentrations, pH, cell density, and age. Different cell lines and different fermentation conditions can produce significantly different glycosylation patterns. Since different glycoforms have the potential to have different biological properties, the ability to monitor and control glycosylation during production is critical to the quality of a biopharmaceutical molecule.
Challenges
There are many glycosylated therapeutic proteins currently available as marketed drugs, five of which are blockbusters and many more are in development. For producers of these proteins, controlling glycosylation is an extremely important element in developing and manufacturing. Indeed, monitoring particular characteristics throughout all stages of a protein´ life-cycle results in greater knowledge of the protein and more predictable process parameters.
The FDA´ Process Analytical Technologies (PAT) initiative encourages companies to build in controls at design of experiment to achieve these optimal characteristics. However, there are several challenges in monitoring glycosylation in an effective and timely manner.
Conventional methods for analyzing glycosylation, such as HPLC and mass spectrometry, are generally accurate and flexible, but they all require 1) significant scientific expertise, 2) purification of the protein and significant sample preparation, 3) use of more than one technique for a complete analysis, and 4) they usually require days or weeks to complete.
These issues make the use of such technologies impractical for high-throughput monitoring of glycosylation during process development and manufacturing. They also make it difficult for the average research laboratory to study the roles of glycosylation on biological properties.
Lectin Array Technology
Lectins are a family of carbohydrate-recognizing proteins that are classified into a number of specificity groups based on the monosaccharides for which they exhibit the highest affinity. Procognia (www.procognia.com) used lectins to develop an array-based method, U-c fingerprint technology, for analyzing glycans of intact proteins.
A set of 20-30 lectins with overlapping specificities is printed onto a membrane-coated glass slide to produce the array. When glycoprotein mixtures are bound to the lectin array they can be detected with a labeled probe to produce a fingerprint that is characteristic of the sample´ glycan profile.
This fingerprint provides sensitive sample comparisons and, following deconvolution, gives a quantitative glycan profile. Using a knowledge base of lectin-glycan recognition rules, a computational inference engine, and interpolation tools, the fingerprint is deconvoluted to provide quantitative information on the glycan structure.
Automated analysis can be performed on small volumes of either purified samples or samples in cell culture media, allowing analysis of up to 20 samples in parallel in 4-5 hours.
Case Study
U-c fingerprint technology was used to monitor trends in glycosylation during fermentation. Samples taken from two different bioreactors at three different time points (day 8, day 10, and day 12) were analyzed directly from cell culture media.
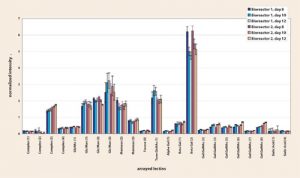
The supernatant samples were diluted with sample binding buffer to a final concentration of 30 µ/mL (0.2 µ); 200 µ of the diluted sample was subjected to treatment for Fc-glycan exposure prior to incubation with the lectin array. Binding of the protein to the lectin array was detected using a fluorescently labeled anti-IgG polyclonal antibody. The array was scanned and the fingerprints were produced using proprietary algorithms (Figure 1). The arrayed lectins (X axis) are grouped and labelled according to their specificities.
Subsequent deconvolution of the fingerprints gave quantitative glycoanalysis.
The U-c fingerprint assay developed for the protein of interest provided quantitative data for N-linked glycan structures. A clear difference in the G0/G1/G2 ratios can be seen between samples from the two bioreactors, showing a higher level of galactosylation in bioreactor 2.
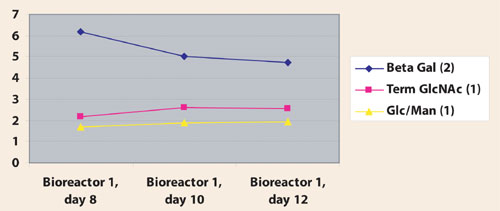
Although there are no significant differences in the interpretation at each timepoint, the fingerprints are more sensitive to changes. At the fingerprint level, differences were seen in epitope presence at different time points throughout a typical fermentation run (Figure 2).
Summary
Procognia´ U-c fingerprint technology provides the capability of monitoring and controlling glycosylation. This technology lends itself to use during the entire life cycle of a glycosylated therapeutic protein.
During clone screening and selection, the desired biological properties can be correlated with glycosylation patterns and can be monitored in all future stages of product development. As only small amounts of unpurified glycoprotein are required, glycosylation monitoring can now begin in much earlier stages of clone screening and selection.
In process development, growth conditions can be chosen by monitoring the effects of choice of media and fermentation conditions on glycosylation. During this stage, monitoring will enable the process engineer to develop a matrix that correlates process changes with glycosylation changes. Such a matrix can be used to make predictions about the effects of future process changes on glycosylation.
Selection of purification protocols can also benefit from the monitoring of fractions after each step. Finally, in-process monitoring of fermentation during manufacture can help determine batch feeding schedules and harvest times and allow the abortion of batches that are not likely to meet release specifications due to aberrant glycosylation.






