The viral vector and plasmid DNA manufacturing market is one of the fastest growing global biotechnology segments. Pegged at $321.3 million in 2017, the market is expected to reach $1.1 billion by 2023, according to Allied Market Research. This growth, which is being driven by an increase in funding for R&D related to gene therapy, could be slowed if gene therapy costs remain high or if gene therapy applications bring unacceptable risks of mutagenesis. Nonetheless, Allied Market Research expects that market players will be presented with lucrative opportunities.
Later this month, viral vectors will be a cornerstone of the 10th Spring Meeting of the International Society for BioProcess Technology select ISBioTech) in Norfolk, VA. One of the speakers, Maurizio Cattaneo, PhD, founder and CEO of Artemis Biosystems, plans to deliver a talk describing how continuous perfusion can achieve an order-of-magnitude increase in lentiviral vector select LV) production. During a preconference interview, he noted that “current adherent cell systems for producing viral vectors are not scalable.”
“Suspension cell systems are scalable,” he added, “but they suffer from the cell-density effect where the LV vector production stays the same independent of how high the cell density is. One million cells/mL gives the same cell productivity as 80 million cells/mL on a volume basis.”
At higher cell densities, vector production faces some challenges. “Due to an unknown limitation or inhibition, the specific productivity of infected batch cultures is found to decrease significantly with increasing cell densities,” Cattaneo stressed. This challenge can be addressed in part by growing cells in a perfusion bioreactor, where continuous supplementation of fresh media and removal of metabolic waste products allow productivity to increase with the cell density.
In the world of producing viral vectors, manufacturers want to make as much as possible. “The main challenge in lentiviral production is the low yield of LV vector,” Cattaneo pointed out. To address that challenge, Cattaneo and his colleagues developed Artemis Biosystems’ VHU Perfusion System, which can make clinical or commercial levels of viral vectors from mammalian cells, including HEK 293 or HeLa cells. By design, the VHU Perfusion System can be easily scaled up or customized, Cattaneo asserted. “Using our VHU Perfusion technology,” he continued, “we can increase the total LV yield 30-fold compared to a batch operation.”
Purification platform
In addition to LV vectors, biomanufacturers and biopharmaceutical companies produce adeno-associated viral select AAV) vectors. This side of vector production will also be covered at the ISBioTech meeting. For example, an AAV-related presentation is expected from Pete Gagnon, chief scientific officer at BIA Separations.
In a preconference interview, Gagnon noted that the most challenging part of purifying AAV comes from an invisible contaminant. “It is host DNA, hiding in the form of chromatin,” Gagnon pointed out. Making purification even more complicated is the high host DNA to AAV ratio—50–100 times higher in comparison to proteins like immunoglobulin G. “A bigger DNA burden going into a process reduces purification performance at every step and often leads to excess DNA in the final product,” Gagnon explained. “[Our] methods remove most of the host-cell DNA in advance.”
The next challenge is that empty capsids—ones without vector DNA—must be removed from the full ones. “The amount of chemical differentiation between full and empty capsids is modest at best,” Gagnon noted. “Developing tools to exploit those differences is genuinely difficult, but improvements are possible if you have enough determination and the right resources.” The goal, Gagnon continued, “is to make density gradient ultracentrifugation unnecessary.”
His presentation, Gagnon said, will describe “two new approaches to sample preparation that enable clinical-quality AAV purification with two chromatography steps.” According to Gagnon, Bia Separations has demonstrated “universal select meaning all serotypes) capture on a cation-exchange monolith that supports repeated sanitization with one molar sodium hydroxide.”
Gagnon plans to unveil “preliminary data on a new method for separating empty and full capsids that offers better performance than the known method of salt-gradient elution on strong anion exchangers.” He also intends to give examples that of a new generation of multidetector HPLC assays that “accelerate process development and validation, and also streamline in-process testing during commercial manufacture.”
In some cases, these new assays can provide results in minutes or hours, Gagnon asserted, rather than the days or weeks that would ordinarily be required. “The main outcome,” he emphasized, “is a fast, universal platform for purification of all AAVs—all serotypes, all cultures, all lysis methods—plus the fast assays to document it.”
Off-the-shelf advances
Many methods of gene therapy involve plasmid DNA. This point will be covered at the ISBioTech meeting by James Brown, PhD, vice president of corporate development at Aldevron. His presentation is expected to emphasize the most pressing plasmid DNA challenges in gene therapy, which are, in Brown’s view, speed of delivery, capacity, and throughput.
According to Brown, “The number of programs in gene therapy is growing, the quantities of plasmid they require are increasing, and the programs are rapidly progressing into and through the clinic.” In reference to the issues with plasmid DNA supply, Aldevron and other companies keep ramping up their capabilities.
“We’ve been expanding our capacity and throughput, resulting in immediate availability” of our GMP-Source plasmid DNA,” he said. GMP-Source plasmid DNA can be used as an ancillary or critical raw material for producing protein and viral candidates, typically for use in early-phase studies. “Generation of a master cell bank—MCB—takes weeks, and scaled-up plasmid is turned around in two to three months,” Brown noted. “As for manufacture of GMP MCB’s and plasmid, lead times—though variable—have also been greatly reduced.”
To add a crucial improvement to the entire process, Brown and his colleagues optimize the production for the intended use of the material. As he explained, “The specifications for a viral vector and the plasmid should be tailored to how these components are being used.”
For example, a plasmid that will be directly injected into a person must meet extremely stringent specifications. On the other hand, if a plasmid is a raw material—being used to make something that will be delivered as a treatment—that plasmid could meet less stringent specifications, but still not impact the safety or efficacy of the final product.
In gene therapies based on AAV or LV vectors, some of the same components—various plasmids—can be used to make a variety of viral vectors. So, Aldevron makes some of these critical materials, including pALD-Lenti packaging plasmids and pALD-X80, which is an adenoviral helper plasmid.
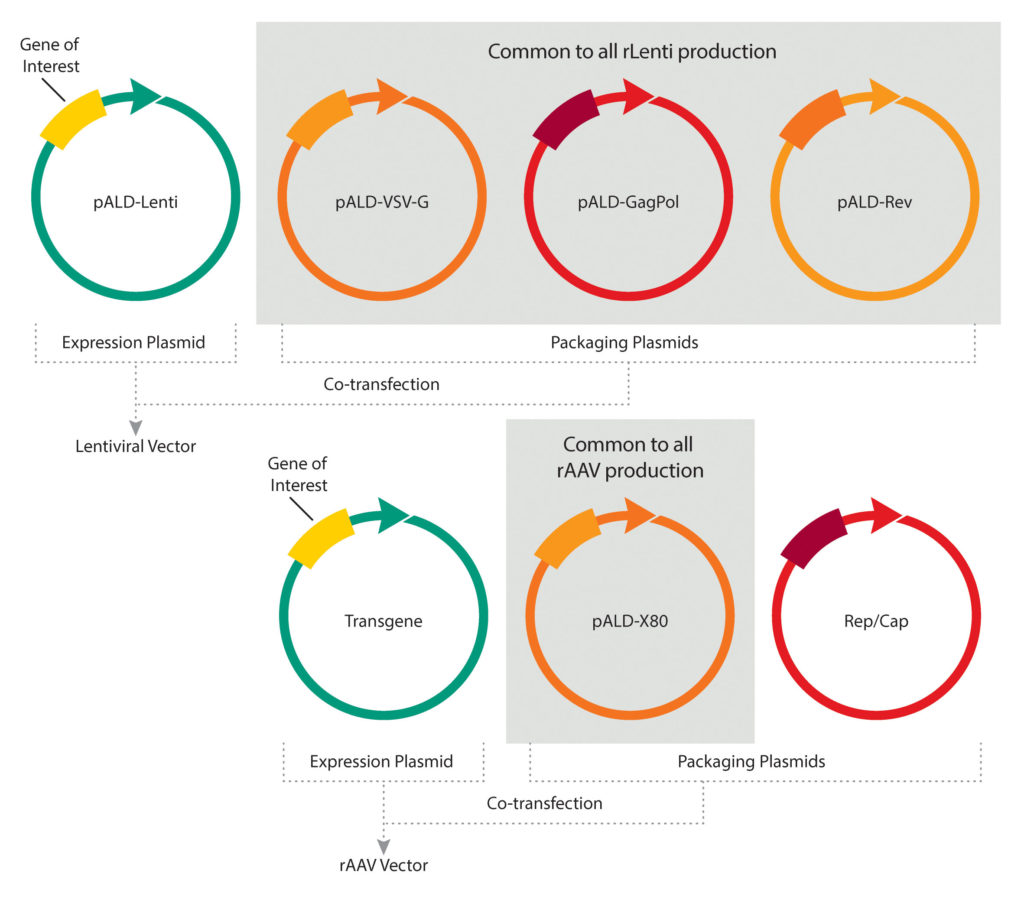
“We have these ready to ship,” Brown declared. “If you want clinical-grade helper and packaging plasmids to make LV or AAV vectors, we can ship that tomorrow.” By manufacturing these more universal plasmids at large scale, Aldevron can “drive down the price and reduce the turnaround time to basically zero,” Brown explained. Additionally, Aldevron offers clients research-grade samples of the off-the-shelf plasmids.
As the experts interviewed here reveal, there are many new tools and techniques for viral vectors that may help biomanufacturers scale-up production, improve purification, or employ off-the-shelf parts. These techniques promise to advance several fields, especially gene-based therapies.
With these new techniques, biopharmas working on new gene therapies can more easily obtain the supplies of viral vectors that are needed. In fact, biopharmas can get more vectors in more forms—all on a faster timetable.
GE Healthcare Life Sciences and Children’s Medical Ink Gene Therapy Manufacturing Agreement
GE Healthcare Life Sciences and Children’s Medical Research Institute agreed to collaborate on the development of new affinity ligands for the purification of adeno-associated viral select AAV) vectors used in gene therapies. The focus of the collaboration is to bring to market specific ligands for multiple AAV types and enhancing the chromatographic separation of AAV-based vectors. This will improve the manufacturing efficiency and scalability of gene therapies, enabling the availability of viral vectors on a global scale, says Olivier Loeillot, general manager, bioprocess, GE Healthcare Life Sciences.
With more than 800 gene therapies currently in clinical trials, there is an increasing demand for the raw materials needed in the manufacturing process of viral vectors. AAVs are viral vectors used in more than 70% of the in vivo gene therapy clinical trials. According to GlobalData, the 2025 gene therapy in vivo therapeutic market is expected to reach $32 billion with an estimated compound annual growth rate of 105% between 2019 and 2025.
The collaboration combines the expertise from the latest available research on AAVs with application testing, advancing a comprehensive understanding of the clinical functionality and the commercial opportunities of AAV-based gene therapies, continues Loeillot. Children’s Medical Research Institute will share with GE Healthcare Life Sciences AAV capsid variants targeting different tissues. GE Healthcare Life Sciences will then design and test ligand prototypes, which Children’s Medical Research Institute will assess. Based on the performance results, GE Healthcare Life Sciences will manufacture and commercialize novel improved AAV affinity ligands.
Leszek Lisowski, PhD, lead gene therapy scientist at Children’s Medical Research Institute, notes, “Bringing the fruits of our work to the patients requires a joint effort between academia and the industry. The collaboration with GE Healthcare Life Sciences will allow us to expedite the development of novel clinical options at a lower cost.”
NxGEN Vector Solutions and ImmunoCurex Merge
NxGEN Vector Solutions merged last month with ImmunoCurex, a gene therapy company focused on generating programmed death ligand 1 select PD-L1) polypeptides for AAV gene transfer. NxGEN Vector Solutions will gain a second AAV gene therapy platform—an immunosuppressive therapy to treat immune or inflammatory diseases or conditions—and plans to continue to develop the technology. NxGEN Vector Solutions is collaborating with Robert Jarvik, MD, the inventor of the artificial heart and founder of Jarvik Heart, and Douglas McCarty, PhD, senior director of vector development at Pfizer, on this platform.
Despite lifelong immunosuppressive therapy, chronic cardiac rejection, which is characterized by interstitial fibrosis, vascular occlusion, cardiac hypertrophy, and progressive dysfunction of the graft, is the main impediment to long-term transplant, notes Susan M. Faust, PhD, CEO of NxGEN Vector.
“ImmunoCurex previously showed in an animal model of chronic cardiac allograft rejection that combined transient immunosuppression and targeting of programmed cell death protein select PD-1) through AAV-mediated PD-L1 gene transfer can inhibit pathologic donor-reactive T- and B-cell responses in vivo and block chronic allograft rejection,” she says. “This strategy attenuates graft fibrosis to a level comparable to an animal model of allograft transplantation whose grafts remain free of cardiac rejection and provides a new treatment strategy to overcome a critical challenge to long-term transplant success.”
A current limitation to the PD-L1-AAV vector, however, is that a soluble form of PD-L1 may exist following cleavage of the membrane-bound PD-L1 protein by matrix metalloproteinase-13, continues Faust. The cleavage product of membrane-bound PD-L1 results in a soluble PD-L1 protein that may allow cancers to evade the host immune system. Thus, elimination of the soluble form of the protein as a therapeutic would be preferred, and there is a dire need to provide localized PD-L1 expression to treat chronic cardiac heart rejection, she adds.
“With human translation and commercialization in mind, NxGEN Vector Solutions will explore the feasibility of an optimized PD-L1 variant,” explains Faust. “[The variant’s construction should] eliminate the soluble form of PD-L1 while maintaining PD-L1’s function as an immune modulator and providing localized expression of PD-L1 following AAV gene transfer in animal models of cardiac rejection and, subsequently, heart failure.”
Vigene Biosciences Opens Global Headquarters
Vigene Biosciences opened its 71,000-square-foot global headquarters in Rockville, MD. The new facility boasts three campuses and 10 GMP suites, including those for viral vector and plasmid biomanufacturing. It combines novel manufacturing technologies and high-yield production cell lines operating under cGMP and BSL-2 standards.
As a contract development and manufacturing organization, Vigene is committed to making gene therapy affordable. It provides viral-vector-based gene delivery services and products for research and clinical applications. The company says that it intends to streamline virus production from early conceptual stages to commercial manufacturing.
“The grand opening today is a major milestone for Vigene. The new facility will enable us to serve our clients faster and better. Six years ago we had two employees and 300 square feet of space. Now we have 100 employees. We expect to double or triple our staff and workforce over the next two or three years,” said Zairen Sun, PhD, founder, CEO, and board chairman of Vigene, adding that “Vigene is one of the few gene therapy CDMO companies in the world which offers research-grade, pre-IND, IND, and commercial-grade viral vectors and plasmid DNAs.”
Gina Hann, the founder of the nonprofit organization Batten Hope, was Vigene Biosciences’ grand opening special guest. “I want everyone in this building to understand why the work that you are doing is so, so important,” she said. “This is a great day for all of us and especially for all of the children that face rare disease like my son Joseph. … I just wanted to say thank you so much for your hearts and hard work and all of your great innovation. You are absolutely changing the world for the better. …You guys are making that one shot for our kids. Thank you.”
“Vigene Biosciences has been an ardent supporter of Batten Hope and a stalwart advocate for the patient community,” added Jeffrey Hung, PhD, chief commercial officer of Vigene. “The company’s expanded production capacity and our focus on increasing manufacturing efficiency will help drive down therapy costs for families like the Hanns.”






