Fungal communities (mycobiota) constitute a subset of the human microbiome that can trigger an immune response, but little is known about their presence or functional role in human cancers. Two independent studies published in the journal Cell, report converging results that establish the association of specific fungal species with diverse human tumors. The evidence indicates mycobiota signatures could predict, diagnose, or monitor the progression of the dreaded disease.
One of the studies was reported by scientists led by Iliyan Iliev, PhD, an associate professor of immunology and microbiology at Cornell University and Anders Dohlman, a PhD student in biomedical engineering at Duke University. The other study was reported by scientists from the University of California, San Diego (UCSD) led by Rob Knight, PhD, a professor of pediatrics, computer science, and engineering, and the Weizmann Institute in Israel led by Ravid Straussman, MD, PhD, a professor of molecular and cell biology.
“The existence of fungi in most human cancers is both a surprise and to be expected,” said Knight. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
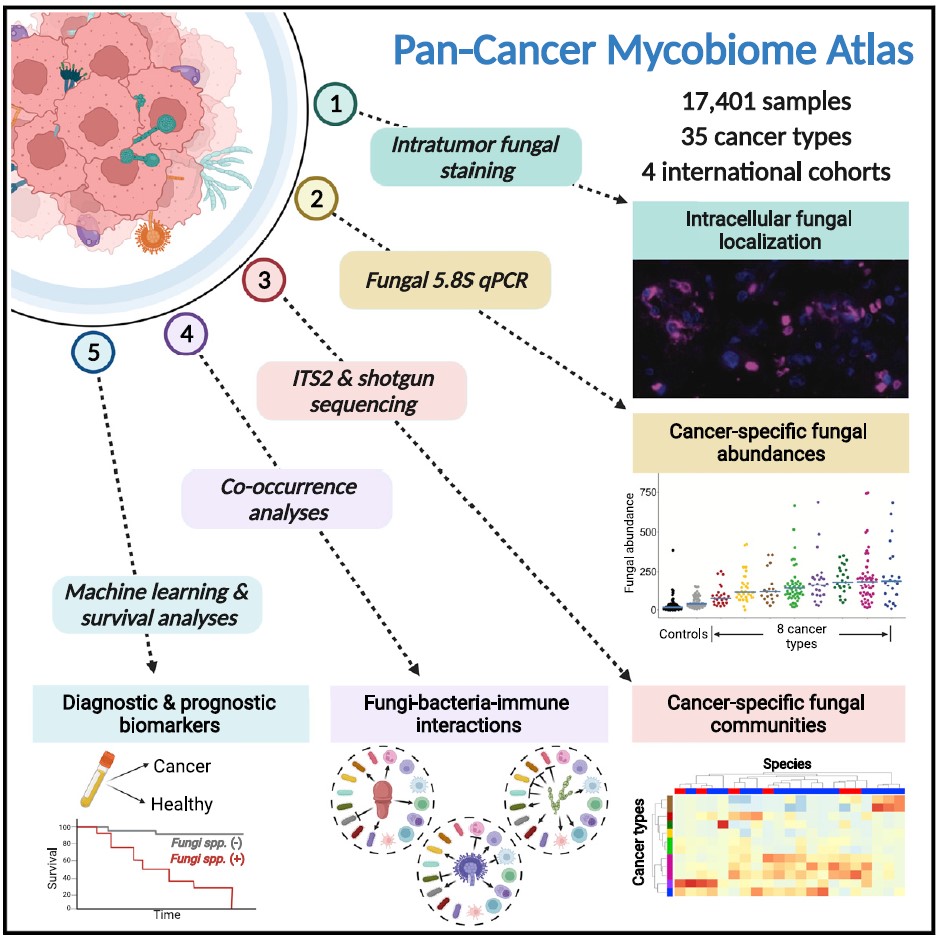
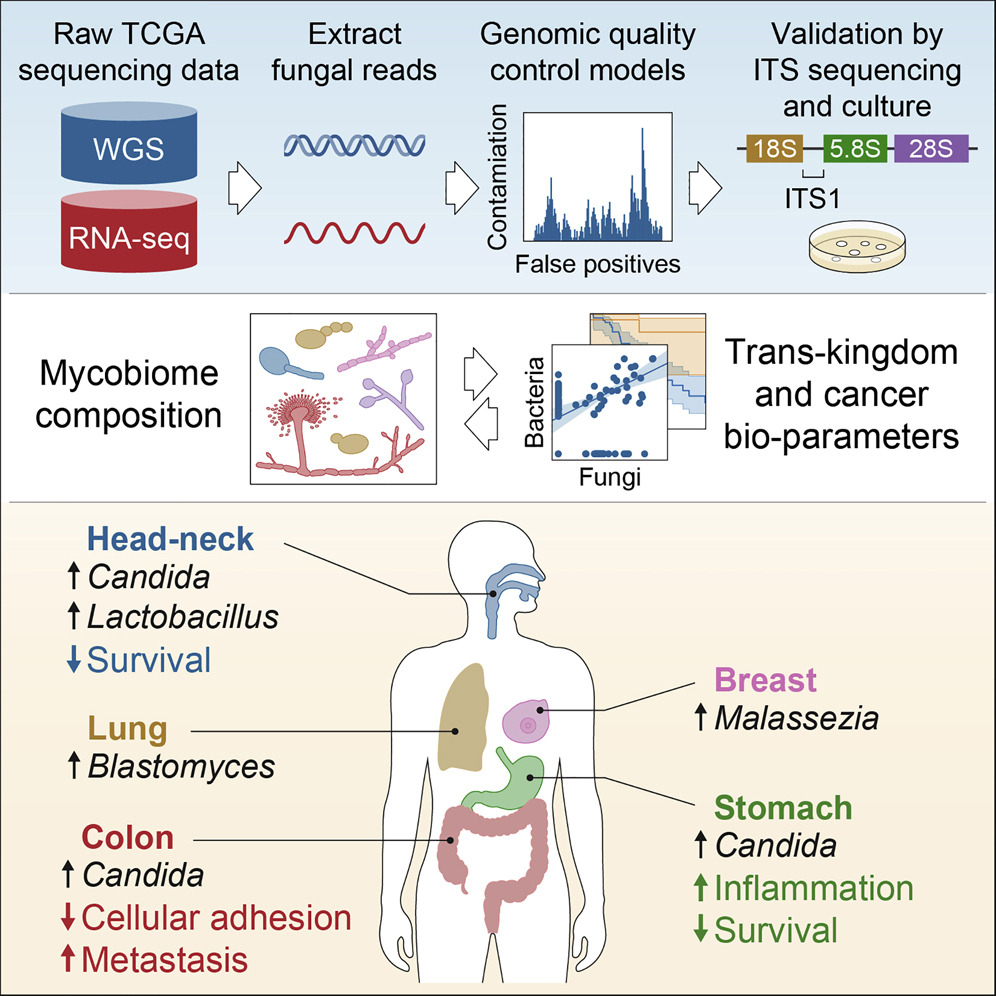
The study from the Cornell and Duke team analyzed several types of cancers using human tumor samples and detected up to one fungal cell for every 10,000 cancer cells. The team also identified individual fungal species in the tumor-associated mycobiota following a rigorous protocol of decontamination and sophisticated computation to eliminate false positives.
In cancers of the gut, they found live, transcriptionally active Candida species. “Candida species were associated with gastrointestinal tumors (in head neck, stomach, and colon cancer),” said Iliev. “We validate these findings in adenocarcinomas using culture dependent methodology as well as in an independent cohort. We also find Blastomyces species associated with lung tumors and Malassezia species associated with breast tumors.”
The researchers cataloged fungal species and their associations with different cancers by analyzing the Cancer Genome Atlas, a genomic database of human tumors. Using ITS (Internal Transcribed Spacer) sequencing, a method that sequences a region of ribosomal RNA, they confirmed the presence of the fungal species in the tumor samples.
“We find some interesting and potentially important associations between Candida species and tumor outcomes that can serve as valuable prognostic indicators,” said Iliev.
Iliev’s team found the presence of Candida DNA associated with various gastrointestinal tumors to be predictive of reduced survival. Whereas high rates of Candida were linked to the expression of pro-inflammatory factors in stomach cancers, in colon cancers the fungus predicted metastasis and reduction of cell adhesion.
The researchers often detected DNA from the same Candida species in both gastrointestinal tumor samples and matched blood samples from the same patients.
“These data are exciting because they lay the foundation for simple, inexpensive tests for Candida DNA that can more precisely delineate prognosis for gastrointestinal cancers and augment standard tumor DNA biopsies to enable early detection of these cancers before other signs are present,” said co-author Steven Lipkin, MD, PhD, professor of medicine at Weill Cornell Medicine.
Iliev’s team found no fungal sequences in brain tissue tumor samples, which is expected as fungal brain infections are frequently lethal. The outcome, however, validated the investigative strategy.
“Additional laboratory data and tumor expression patterns suggest that these fungi are associated with the tumor mucosa but are not living inside the tumors. This is a key point as intracellular organisms can lead to a very different type of immune response,” said Iliev.
The team is currently working on a model system to better understand the interactions between tumor-associated fungi and the tumor microenvironment. In addition, the team also intends to investigate the potential contribution of tumor-associated fungi in promoting malignancy and related inflammation.
“I was excited to see the paper from Iliev’s group is in agreement with many of our findings—mainly that fungi are a part of the tumor microenvironment,” said co-lead author of the study, Lian Narunsky Haziza, PhD, a postdoctoral fellow in the Straussman lab at the Weizmann Institute in Israel. “That we used different approaches for the analysis and decontamination of the data and still got to similar conclusions strengthens the findings. I hope these studies will increase focus on the tumor mycobiome.”
Gregory Sepich-Poore, an MD/PhD student at UCSD and co-lead author of the study said, “I am encouraged that an independent study has validated many of the findings we made in our paper, especially the existence of cancer type-specific fungi, underscoring the validity and generalizability of using different methods.” Sepich-Poore and Knight have co-founded a company, Micronoma, that is developing microbial biomarkers in blood and tissues to diagnose and treat cancers.
“[The Iliev study] demonstrated similarities between fungi identified in tumor tissues and matched blood samples,” added Sepich-Poore. “This supports a hypothesis we also made in our paper: tumor tissues are a substantial source of blood-derived fungal (and bacterial) DNA. This suggests the potential utility of cell-free microbial DNA as a minimally invasive diagnostic marker for cancer.”
Using fungal histological staining of tissue microarrays, the Knight and Straussman team showed the presence of fungi in tumors, and frequent spatial association of fungi with cancer cells and immune macrophages. In addition to analyzing the mycobiome, the group also compared the tumor mycobiome with matched bacterial and immune cell communities associated with the same tumors. This revealed the relations among fungal, bacterial, and immune components associated with tumors were often permissive, or tolerant to each other’s growth, rather than competitive.
Similar to the Iliev study, Knight and Straussman’s study noted that whether fungi associated with specific tumors promote cancer, remains to be investigated. Straussman said, “The finding that fungi are commonly present in human tumors should drive us to better explore their potential effects and re-examine almost everything we know about cancer through a ‘microbiome lens’.”