By Delthia Ricks
About 48 hours after a Seattle woman received the first dose of an experimental vaccine against SARS-CoV-2, scientists in China—not to be outdone—announced that they had regulatory approval to launch an ambitious vaccine trial as well. The Seattle volunteer received a messenger RNA vaccine that Moderna Therapeutics developed to prevent infection with the pandemic virus. The Chinese scientists, representing CanSino Biologics, described their plans to evaluate a recombinant vaccine.
The worldwide scientific mobilization to produce a SARS-CoV-2 vaccine has become a race against time—and an escalation in the heated rivalry among competing companies. All are scrambling toward the same goal: producing a vaccine against a viral menace that emerged in central China late last year and is now circling the globe.
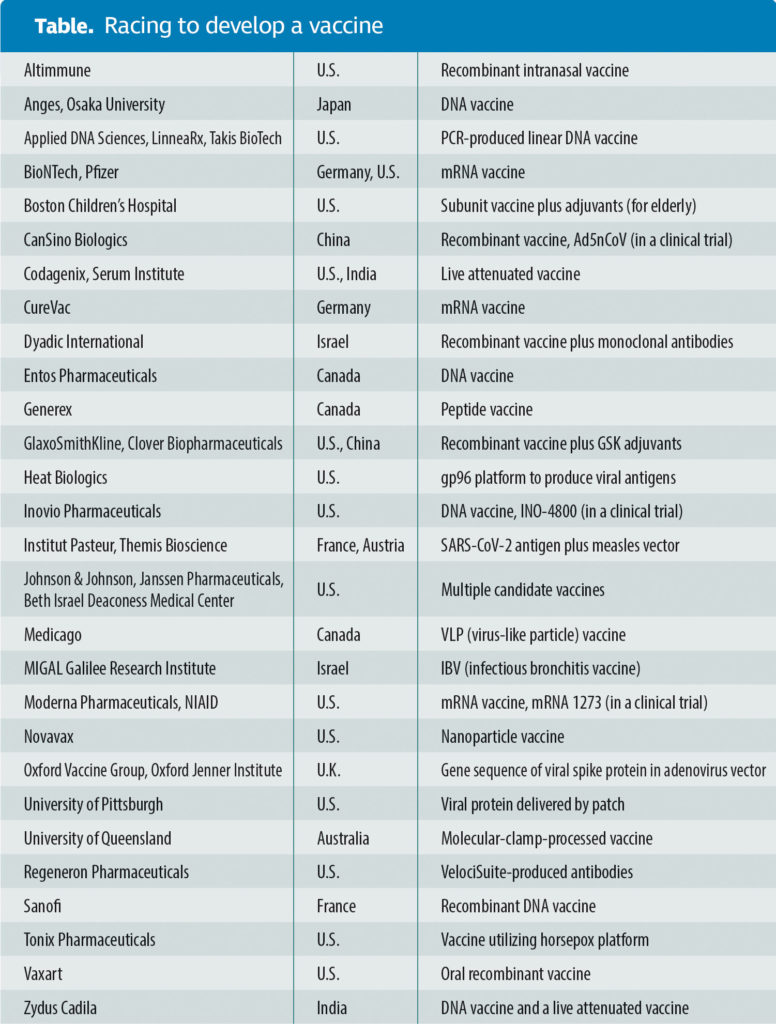
At the time of this article’s preparation, in mid-April, more than 2 million people around the world had become infected with the novel coronavirus, and deaths had already breached the 100,000 mark, with no end to the pandemic’s exponential growth in sight. It will be months, public health officials say, before it is known whether either of the vaccines entering clinical trials will be widely used to effectively immunize populations against SARS-CoV-2. Multiple other candidate vaccines are being studied by research teams globally, and several are set to be launched into clinical testing within the next few weeks to months.
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said in a statement. He added that it is impossible to give a precise date when a vaccine will be ready for what will amount to tens of millions of immunizations.
Developers looking to make the most of a head start
The first U.S. candidate vaccine to reach clinical trials—mRNA-1273—was developed by Moderna Therapeutics of Cambridge, MA, in collaboration with scientists at the Vaccine Research Center, a division of the NIAID. The experimental vaccine targets a prefusion viral spike protein, the molecule that binds to ACE2 receptors in lung cells to initiate infection. The vaccine entered clinical testing on an unprecedented timetable. It took just 42 days from the time of SARS-CoV-2’s genetic sequence being posted online by Chinese researchers in January to the moment when the first vaccine dose was injected at Kaiser Permanente Washington Health Institute on March 16. The NIAID is overseeing clinical testing.
If the vaccine is eventually approved by the U.S. Food and Drug Administration (FDA), it could become a first of its kind—a genomic vaccine—to be rolled out for mass vaccinations.
Tal Zaks, MD, PhD, chief medical officer at Moderna, said the newly launched clinical study marks a significant first step toward development of an mRNA vaccine against SARS-CoV-2. Zaks underscored that he and his colleagues at Moderna and NIAID expect key clinical information about safety and immunogenicity to emerge from the first round of clinical testing.
“The potential advantages of an mRNA approach to prophylactic vaccines,” Zaks pointed out, “include the ability to mimic natural infection to stimulate a more potent immune response.”
Moderna and federal scientists skipped animal testing because “it wasn’t on the critical path to getting mRNA-1273 to a clinical trial,” a Moderna spokeswoman said. Doing so also quickened the pace to clinical testing. The research team’s preclinical research, meanwhile, compellingly suggested that an mRNA vaccine will generate a robust immune response.
“Multiple mRNAs encoding for multiple viral proteins can be combined in a single vaccine, permitting production of complex multimeric antigens that are more difficult to achieve with traditional technologies,” Zaks explained. “Our ability to design our antigens in silico allows us to rapidly produce and test antigens and move vaccine development candidates quickly into the clinic.”
The open-label Phase I trial started March 16 with a plan to enroll 45 healthy adult volunteers between the ages of 18 and 55, and to evaluate different doses of the experimental vaccine for safety and the ability to induce an immune response in participants. This is the first of multiple steps in the clinical trial process for evaluating the potential benefit of the vaccine. Fauci noted the study was “launched in record speed.”
Yet even as Moderna researchers and their NIAID collaborators marked a milestone for the swiftness with which they produced mRNA-1273, China’s CanSino Biologics in Tianjin was in hot pursuit of the same goal: beginning a vaccine clinical trial as a first step toward a SARS-CoV-2 immunization. Details posted on the Chinese Clinical Trial Registry explain that the Phase I clinical study will involve testing the experimental product in adult residents of Wuhan, China who are 18 to 60 years old. The city was the epicenter of China’s SARS-CoV-2 outbreak, which exploded into a worldwide pandemic. Cases in China subsided as the pandemic virus moved around the world.
The vaccine, which is called Ad5-nCOV, is a recombinant product that utilizes CanSino Biologics’ replication-defective adenovirus vaccine platform. Although Ad5-nCOV represents a technological approach distinct from that taken by mRNA-1273, it also represents an attempt to raise antibodies against the SARS-CoV-2 spike protein.
All told, 108 participants will be included in the Ad5-nCOV trial, according to the registry, and divided into three groups, each of which will receive a different dose of the recombinant product.
Developers trying to break out of the pack
Candidate vaccines are under development at more than two dozen academic centers and biotechnology and pharmaceutical companies throughout the United States and abroad. In Germany, two biotechs, CureVac and BioNTech, are also working on mRNA vaccines. The latter is collaborating with Pfizer in the United States.
The types of SARS-CoV-2 vaccines under development worldwide are mostly genomic, DNA or RNA. However, recombinant products similar to the platform pursued by CanSino Biologics are under investigation as well. Additional countries hoping to mitigate future SARS-CoV-2 morbidity and mortality with a vaccine include Israel, France, India, Canada, and Australia, to name a few.
Although vaccines of all kinds have remained elusive for countless viruses, ranging from human immunodeficiency virus (HIV) to the severe acute respiratory syndrome–related virus that emerged in 2003 (SARS-CoV-1), the fast tempo of the current quest has been unmatched since the race to develop a vaccine against polio more than 65 years ago.
Björn Meyer, PhD, a virologist who specializes in the study of RNA viruses at Institut Pasteur in Paris, emphasized that because human populations have no immunity to the newly emerged coronavirus, time is, necessarily, of the essence. “The speed at which SARS-CoV-2 is able to spread is remarkable,” he observed. “It is not surprising for a respiratory virus to do this, given the reproductive number of such viruses.”
The novel coronavirus differs in subtle, yet significant ways compared with previous members of the β-coronavirus family, SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV). Meyer continued: “Those viruses seem to primarily infect the lower respiratory tract; therefore, they might be slightly more difficult to spread between humans. The new SARS-CoV-2, however, seems to replicate well in the upper respiratory tract, grows there quickly to high viral loads, and is able to spread between humans, who transmit it simply by droplets produced during breathing and coughing.”
To speed vaccine development, the Coalition for Epidemic Preparedness (CEPI), an Oslo-based nonprofit, distributed grants to several biotechnology companies and academic centers in January. Moderna, one of the recipients, has not disclosed the amount it received. However, CEPI’s hope is to expedite the development of one or more SARS-CoV-2 vaccines by emphasizing new vaccine strategies, such as mRNA technology, or by supporting vaccine approaches already developed for pathogens that cause diseases similar to coronavirus disease (COVID), such as SARS and MERS.
David B. Weiner, PhD, the molecular immunologist who directs the Wistar Institute’s Vaccine and Immunology Center in Philadelphia, PA, describes the global vaccine pursuit as an all-hands-on-deck moment in the science of vaccinology. In many ways, it is the biological equivalent to the Manhattan Project of World War II when the most celebrated minds in physics helped harness the use of atomic energy. With respect to SARS-CoV-2, multiple teams are working on numerous types of vaccines, Weiner said.
“This is not about competition,” he insisted. “We are all in this together.”
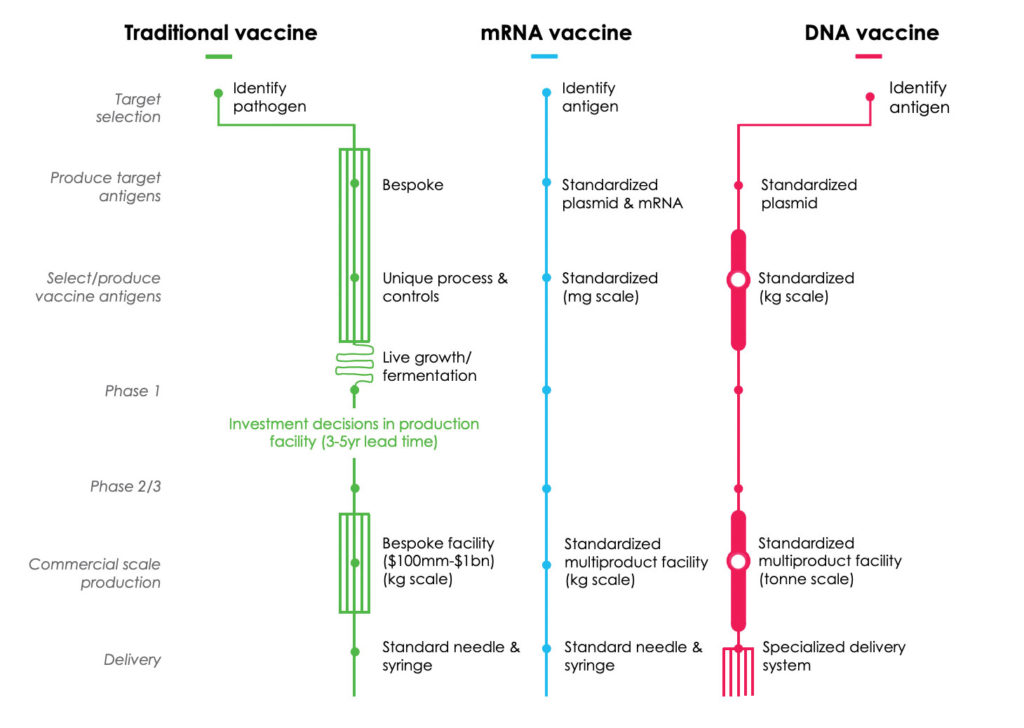
 Weiner conducted research in the 1990s that laid the foundation for exploiting DNA’s versatility as a vaccine. He explained that CEPI chose to fund certain vaccine strategies, such as his DNA vaccine and approaches like Moderna’s mRNA-1273 vaccine, because these “technologies are designed for rapid deployment.” He added, “All of these technologies were chosen because they are fast and have conceptual advantages.”
Weiner conducted research in the 1990s that laid the foundation for exploiting DNA’s versatility as a vaccine. He explained that CEPI chose to fund certain vaccine strategies, such as his DNA vaccine and approaches like Moderna’s mRNA-1273 vaccine, because these “technologies are designed for rapid deployment.” He added, “All of these technologies were chosen because they are fast and have conceptual advantages.”
A candidate vaccine known as INO-4800, a DNA product produced by Inovio Pharmaceuticals, in Plymouth, PA, is based on technology developed in Weiner’s laboratory. Inovio launched its U.S. human clinical trial in April. Clinical testing is expected to involve 30 volunteers. Trials of INO-4800 also are planned to launch in May in South Korea and China. Those investigations, however, will be substantially larger with an aim to enroll about 3000 participants.
DNA vaccines are composed of double-stranded plasmids whose sequences are designed via computer and are meant to produce a specific immune response in the body. Weiner said composing each segment is like fitting together Legos. Vaccination with the proprietary DNA vaccine requires a special handheld “smart” device, called the CELLECTRA, which releases a brief electrical pulse to usher the DNA vaccine under the skin.
If all proceeds as planned, Inovio officials hope to produce up to 1 million vaccine doses by the end of the year. The company received a $9 million CEPI grant to support its preclinical and clinical research. It also was awarded a $5 million grant from the Bill and Melinda Gates Foundation to accelerate testing of its handheld device.
In Tübingen, Germany, a biopharmaceutical company called CureVac is, like Moderna, developing an mRNA vaccine. CureVac’s head of communications, Thorsten Schüller, told GEN that the company is on track to launch ITS vaccine into clinical trials by this summer. The European Commission awarded the company $86.2 million (€80 million) to aid its research and production.
CureVac was in the spotlight recently after a German newspaper, Welt am Sonntag, claimed the Trump administration tried to poach vaccine scientists from the company and to acquire CureVac’s mRNA vaccine exclusively for the United States. CureVac has denied the claim.
Schüller said the company’s key focus is its SARS-CoV-2 vaccine. Behind this vaccine is a complex technology that Schüller explains in deceptively simple terms: “With our mRNA technology, we instruct the human body to produce its own vaccine.”
CureVac’s SARS-CoV-2 vaccine isn’t its first using mRNA technology. The company has been testing its lipid nanoparticle mRNA rabies vaccine in human clinical trials. Results, Schüller said, have exceeded expectations, a sign that the field of vaccinology is on the brink of a new era, one that features the newly emerging mRNA vaccine. “We have indeed seen a long-lasting immune response in our preclinical studies with our mRNA-based vaccines,” Schüller said.
“In our ongoing clinical study,” he added of the rabies mRNA vaccine, “available data appear to confirm that in humans.”