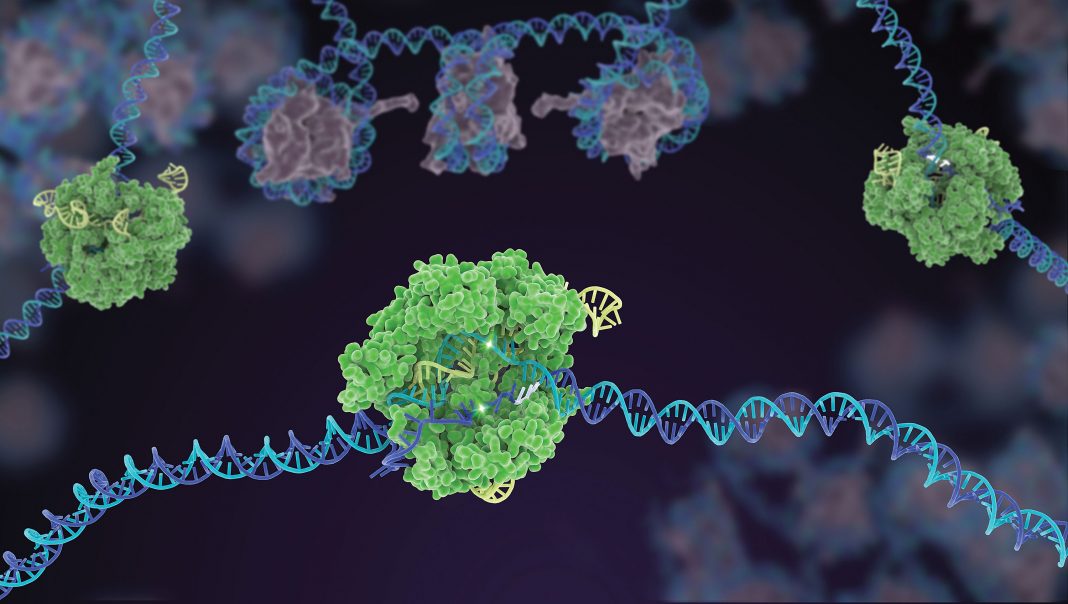
Genome engineers have a strange way of expressing gratitude. After they discover the usefulness of the CRISPR-Cas9 enzyme that was so generously bestowed by Nature, they start finding fault. CRISPR-Cas9, they grumble, is responsible for too many off-target effects. It’s too large to fit within commonly used viral vectors. It’s useless if it strays too far from suitable protospacer-adjacent motif (PAM) sequences. Or it’s always “on,” everywhere, lacking in spatiotemporal control.
Genome engineers might complain endlessly except for one thing: the work of protein engineers. Whenever plain, old CRISPR-Cas9 demonstrates flaws that would prevent desirable applications from being realized, protein engineers get busy. Using techniques such as rational design, directed evolution, and domain fusion, they start developing improved CRISPR-Cas9 variants.
In recent months, protein engineers have reported progress in several Cas9 reengineering projects. For example, combining evolutionary information and directed engineering, scientists based at Tsinghua University in China sought to develop Cas9 variants with expanded PAM recognition capabilities. In a Nature Communications article that appeared online last February, the scientists claimed to generate a “panel of chimeric Cas9 variants accessible up to 1/4 of all the possible genomic targets in mammalian cells.”
Using a directed evolution approach, the University of California, Berkeley, lab of David Savage, PhD, developed Cas9 variants that are smaller than the usual Cas9 derived from Streptococcus pyogenes. The Savage lab’s protein engineering method, called Minimization by Iterative Size Exclusion Recombination (MISER), was described at ASCB/EMBO meeting last December. MISER permits the systematic creation of all possible contiguous deletions of a given protein sequence. According to the Savage lab, MISER enabled the directed evolution of “dramatically smaller proteins that still retain parental function.”
The Savage lab also reported that it used circular permutation, a domain fusion technique, to give Cas9 an “on” switch. The technique involves taking the amino acid string of the Cas9 protein and cutting it, switching the order of the two segments, and then allowing it to fold into a new 3D configuration.
In a Cell article that appeared last January, members of the Savage lab reported that they had used circular permutation to generate protease-sensing Cas9s (ProCas9s). The ProCas9s didn’t just rearrange Cas9’s internal components. They included a sort of molecular lock that connected newly joined segments, a lock to which a key, a protein-cutting enzyme, could be supplied.
“ProCas9s can sense a wide range of proteases, and we demonstrate that ProCas9 can orchestrate a cellular response to pathogen-associated protease activity,” the article’s authors wrote. “Together, these results provide a toolkit of safer and more efficient genome-modifying enzymes and molecular recorders for the advancement of precision genome engineering.”