Biotechnology is generally agreed to have originated in 1973, when Stanley N. Cohen and Herbert Boyer reported that they had engineered Escherichia coli to incorporate and express recombinant DNA. Just seven years later, in 1980, Wall Street saw the first initial public offering (IPO) of a biotech company, Genentech, based on recombinant human insulin. In that IPO, Genentech raised $35 million—about $112 million in 2020 dollars.
At the time, this was a stunning figure, but it would be unremarkable for a biotech IPO today. Now, 40 years later, healthcare stands transformed by the thriving science and business of biotechnology, where there is nothing remarkable about doctors prescribing recombinant proteins or antibodies, or about payors forking over tens or hundreds of thousands of dollars for these high-tech treatments.
Some technologies that have emerged and altered the landscape in recent years include immunotherapy, CRISPR-Cas9 gene editing, and chimeric antigen receptor (CAR) T-cell therapies. Now, another platform technology is maturing from the research laboratory to commercial viability.
In 2017, the U.S. Food and Drug Administration (FDA) approved the first directly administered gene therapy for mutations of a specific, disease-related gene. That product, Luxturna, marketed by Spark Therapeutics, delivers a functioning copy of the RPE65 gene to retinal cells using an adeno-associated virus (AAV) as a vector to treat a genetic form of blindness.
This advance has injected new energy into biotech startups seeking to capitalize on gene therapy. Like synthetic insulin, gene therapy is expected to have a transformative influence on medicine. A closer look at three of 2020’s crop of gene therapy IPOs reveals companies following in Genentech’s footsteps by bringing “blue sky” science into everyday clinical practice—while also turning a profit.
Solving the delivery problem
Hearing disorders have been a challenge for drug developers because of the delivery challenges of the ear. It is an enclosed space, where drug regimens can easily damage sensitive structures. Great pains must be taken during drug administration.
This drug delivery challenge has been taken up by Akouos, a company that develops gene therapies for people with hearing loss. The company envisions a gene therapy that could be administered in a single, one-time dose and provide a lifelong benefit. Akouos issued an IPO in June, pricing above range at $17 per share, for gross proceeds of $244.4 million—just about double Genentech’s inflation-adjusted IPO.
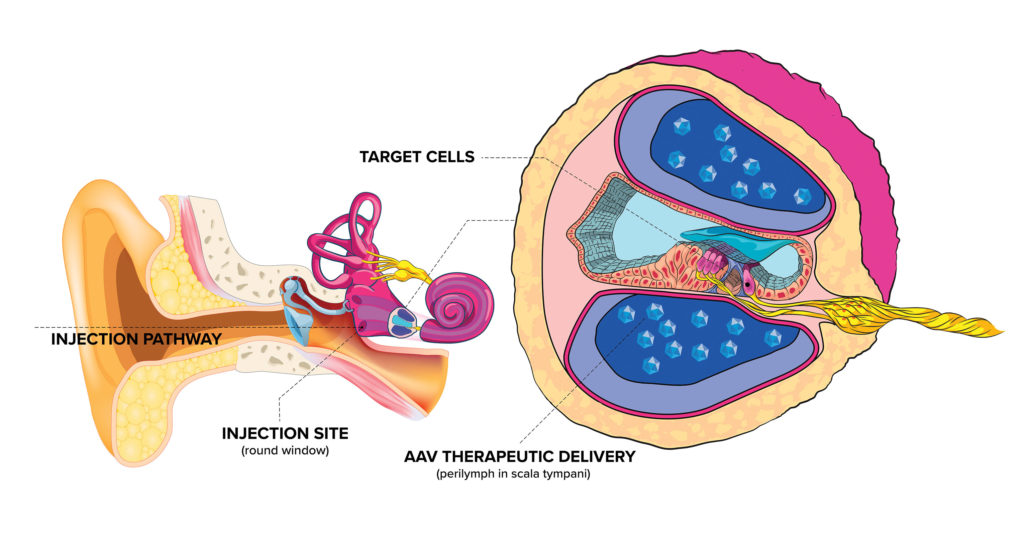
“We’ve developed a novel delivery approach including a device that surgeons can use to get vector into the inner ear fluid space,” says Akouos’ co-founder and president, Manny Simons, PhD. The custom device is paired with an AAV vector developed at Massachusetts Eye and Ear called Anc80 that transduces cells of the inner ear at a higher rate than naturally occurring AAV, and with a lower dose for comparable levels of gene expression.
“This is important because the inner ear is a fixed volume space where there’s a cap on how much vector you can put into it,” explains Simons. “It opens up an entire new target landscape within genetic and other forms of hearing loss that were previously thought to be inaccessible with AAV.”
Akouos’ pipeline includes programs for hereditary and nonhereditary hearing disorders. The company’s lead program aims to restore function of the otoferlin gene (OTOF). Mutations in OTOF interfere with the ability of the sensory cells of the ear to release neurotransmitters in response to sound stimulation to activate auditory neurons.
Akouos released preclinical data on that program in 2019 and is working with the FDA toward human clinical trials. The company is also working on a nonhereditary disorder, vestibular schwannoma, using the same AAV vector, Anc80. For that disorder, the genetic payload is designed to enable cells in the inner ear to produce a protein that would inhibit growth of the tumor, ideally allowing the patient to avoid surgery. Akouos has several other programs covering genetic and nongenetic hearing loss.
Emphasis on manufacturing
Manufacturing challenges in gene therapy development can be more daunting than those in small-molecule drug development, suggests Mani Foroohar, MD, a senior research analyst with SVB Leerink. “The clinical predictiveness of early data for gene therapy is quite robust,” he says. “So, a lot of the attention and focus goes from the clinical data to manufacturing quality and execution processs, things that are a much smaller part of the story in traditional small-molecule drugs.”
Passage Bio is one company leveraging manufacturing expertise to bring gene therapy products to market. Its technology is sourced from the University of Pennsylvania’s Gene Therapy Program. CEO Bruce Goldsmith, PhD, says that the Gene Therapy Program works with Passage Bio to de-risk and differentiate potential gene therapy products for development.
Passage Bio has options to license 17 programs focused on monogenic disorders of the central nervous system (CNS). The company has already decided to move forward with six of those programs, which include GM1 gangliosidosis, Krabbe disease, frontotemporal dementia, and amyotrophic lateral sclerosis. On the strength of these programs, Passage Bio was able to raise $284.4 million in its initial public offering this year.
“What Passage Bio brings to the table,” Goldsmith asserts, “is manufacturing, development, and commercialization expertise” that is relevant to the treatment of CNS disorders.
The company’s areas of expertise include vector selection. Important capabilities for vectors, Goldsmith says, include superior targeting: “This continues to be something that’s very important to focus on, to increase the targeting of the specific cells of interest and therefore increase efficacy and safety.” Goldsmith also notes that vectors should avoid neutralizing antibodies. He points out that Passage Bio is working on improvements such as decreased immunogenicity that might avoid side effects and adverse events.
Although gene therapy is still novel, Goldsmith envisions a future in which it is a routine part of treatment for a range of conditions. “The idea of treating all diseases is going to have to combine gene therapy with gene editing, with very specific targeting and possibly even suppression of existing proteins to make it all work together,” he declares.” I think we’re a long way from there, but the groundwork across multiple companies and academic institutions has certainly been laid.”
Nonviral gene therapy
Generation Bio is another gene therapy company that went public this year. Its IPO in June raised $230 million to advance its lead programs in phenylketonuria (PKU) and hemophilia A. The company plans to file investigational new drug applications by 2022. The company also has a pipeline of preclinical programs for diseases of the liver and retina including Wilson disease, Gaucher disease, and wet age-related macular degeneration.
Generation Bio’s CEO, Geoff McDonough, MD, says that while breakthroughs in AAV-based gene therapy have sparked new interest in the field, enthusiasm had been growing for at least a decade before the recent approvals of two AAV-based gene therapies. There is also reason, McDonough suggests, to be optimistic about alternatives to viral vectors.
“Certainly, many in academia and industry are looking to improve and optimize new generations of viral vectors, and we hope that work bears fruit,” he remarks. “But to dramatically expand the reach and scale of gene therapy overall, we believe nonviral gene therapies are the future.”
AAV-based gene therapy has some shortcomings. It excludes patients with preexisting AAV antibodies. And because antibodies will develop after the body’s first exposure to AAV vectors, AAV-based gene therapy is limited to a single, fixed dose that doesn’t guarantee adequate expression levels.
Generation Bio’s goal is to create a new class of durable, re-dosable nonviral gene therapy for rare and widely prevalent diseases. To that end, the company is working on three technologies.
The first is a closed-ended DNA construct called ceDNA that McDonough says has three times the payload capacity of an AAV vector. The second is a cell-targeted lipid nanoparticle that allows redosing, potentially throughout life, to achieve initial gene expression levels. The third is a capsid-free manufacturing process that would allow for dramatically scaled up manufacture, from thousands to millions of doses.
Generation Bio is developing its platform to deliver genetic payloads that can enable a patient’s own liver cells to produce therapeutic antibodies against infectious and systemic diseases. The platform also has potential beyond gene therapy.
In August, there was some concern in the gene therapy field when BioMarin Pharmaceutical unexpectedly received a complete response letter (CRL) from the FDA about valoctocogene roxaparvovec, the company’s AAV-based gene therapy product for hemophilia A. The agency recommended that Biomarin complete its Phase III study and submit two-year safety and efficacy data to show durability of the product’s effects.
McDonough doubts that BioMarin’s CRL will affect Generation Bio’s hemophilia A program. “To the extent that the FDA requires AAV-based clinical candidates to demonstrate long-term efficacy data, this is likely because current generation AAV-based gene therapy may only be dosed once per patient,” he maintains. “We believe this illustrates the potential value of a nonviral approach that enables repeat administration to increase or extend expression in the event of waning efficacy.”
Just another tool in the biotech toolbox
Gene therapy startups that admire Genentech’s success and longevity may want to follow Genentech’s example in building a diversified product portfolio. After developing recombinant insulin (Humulin), Genentech added a string of recombinant protein products targeting various diseases. Then, expanding outward from recombinant proteins, Genentech began rolling out monoclonal antibody drugs in the late 1990s and 2000s, while continuing to innovate in the area of recombinant proteins and small-molecule drugs throughout the 2000s. Roche ultimately acquired Genentech in 2009 for $46.8 billion. Genentech, which declined to be interviewed for this article, now operates as an independent center within Roche.
According to Foroohar, technology platform agility is an important characteristic of the newer crop of gene therapy companies. “Traditionally, people have thought of gene therapy companies as technology platform companies,” he says. “Specifically, companies whose expertise is around a particular delivery vector. We are seeing newer companies that are increasingly platform agnostic and are able and willing to pursue a number of different modalities, mixing and matching the right tool to the right indication.”
Foroohar believes that gene therapy will eventually become one tool of many in the biotechnology and pharmaceutical technology toolbox. “In the armamentarium of every large-cap pharma and biotech company,” he predicts, “there will probably be at least a few gene and cell therapy programs.”
As the field matures, gene therapy companies will eventually need to find a way to sell once-in-a-lifetime cures in a market built around chronic therapies. “In some ways, it’s been easier for our European counterparts and other single-payer economies,” Foroohar comments. “[They] can rest more assured that the financial benefits of reducing future hospitalizations due to expensive future illness will be captured by the same payor that’s providing the upfront outlay.”
“That is not true here in the United States,” he continues. “There have been a number of quite complicated legal and practical impediments to payment-over-time models or contingent payments or other more innovative reimbursement structures.”
Just as biotechnology has transformed every aspect of our healthcare system over the last 40 years, gene therapy will challenge, disrupt, and overturn our healthcare pricing and reimbursement paradigms as it becomes an increasingly common and routine treatment approach.





