Ingmar Hoerr, Ph.D. CureVac
The Power and Potential of mRNA
Messenger RNA (mRNA) offers a potentially transformative paradigm for developing therapeutics and vaccines. One of nature’s core building blocks, mRNA is responsible for translating our genetic code into functional proteins, providing the blueprint for each cell within our body. By engineering mRNA to carry the right information, it is possible to deliver messages to cells to produce everything from disease-fighting proteins to functional antibodies.
Historically, mRNA was viewed as too unstable to function as a therapeutic technology. However, the underlying technology utilized for developing mRNA therapeutics has undergone extensive development to overcome a number of theoretical and practical obstacles. These advancements paved the way for the first mRNA programs to demonstrate proof-of-concept as a therapeutic technology. Further refinements have enabled mRNA-based drugs to enter the clinic, where the first human studies have demonstrated safety, tolerability, and early evidence of targeted physiologic activity.
Pioneers in the mRNA industry have now assembled a library of clinical and preclinical data, which suggest that mRNA-based therapeutics could fill critical gaps unmet by traditional small molecule drugs, biological treatments, and emerging gene therapies. Additionally, mRNA medicines offer added advantages in that they are easily developed, inexpensive to produce, and efficiently scalable for manufacturing purposes. These benefits, combined with the potential to create mRNA for virtually any protein or antibody, have garnered substantial attention from investors and the pharmaceutical industry alike.
In oncology, for example, there has been significant attention focused on immunotherapeutic approaches and the development of therapeutic vaccines designed to activate the immune system to recognize and destroy tumor cells. Recent human studies of an mRNA candidate evaluated the immunostimulatory potential of a therapeutic vaccine to treat prostate cancer by delivering four different antigens. Following multiple vaccinations, 79% of patients demonstrated antigen-specific immune responses, with 58% of the immune responses being directed against multiple antigens.1
Eliciting an immune reaction with mRNA-based antigen delivery has important implications not only for therapeutic vaccine development, but also for developing prophylactic vaccines targeting infectious disease.
Vaccination is the most effective measure for preventing and controlling disease; however, pursuit for an ideal immunization platform is still ongoing. mRNA-based vaccines combine desirable immunological properties, excellent safety profiles, and flexibility that is not provided by genetic vaccines.
In the quest for an ideal antigenic platform, safety is among the major concerns. First, when compared with plasmid DNA and viral vectors, mRNA maintains a superior safety profile. There is no risk of gene integration, transient expression, or lack of anti-vector immunity. Since mRNA serves as only the minimal genetic construct, it merely harbors the elements required for expressing the encoded protein. While recombination between single-stranded RNA molecules may occur, these instances are rare, as there is no interaction between mRNA and the genome. Recently published data in the peer-reviewed journal Vaccine confirmed an excellent safety profile of an mRNA vaccine, as the stimulation of proinflammatory cytokines was localized at the injection site with no systemic cytokine release.
With respect to efficacy, mRNA-based vaccines benefit in that they do not need to cross the nuclear envelope as opposed to DNA. When compared with peptides, mRNA vaccines lack
MHC haplotype restriction. Additionally, mRNA binds to pattern recognition receptors, indicating mRNA vaccines may be designed to self-adjuvante, a property which peptide- and protein-based vaccines lack.
Of particular importance the effectiveness of vaccine-induced adaptive immunity is critically dependent on the level of the initially triggered innate immune responses. mRNA poses intrinsic adjuvant activity via its recognition by endosomal Toll-like receptors (e.g., TLR7, TLR8) and cytoplasmic sensors (e.g., RIG-I or MDA-5). Upon these receptors being triggered, proinflammatory cytokines are produced, which is a prerequisite of a successful immunization.
Preclinical data of mRNA vaccines in various animal models for different indications have demonstrated protective immune responses similar or superior to those triggered by commercially available vaccines. Specifically, studies have demonstrated the immunostimulatory capacity of mRNA vaccines via increased numbers of immune cells activated within the draining lymph nodes.
In addition to possessing an excellent safety profile and potential immunostimulatory properties, mRNA is also a highly flexible technology platform for expressing specific antigens. Studies have shown mRNA-based vaccines’ ability to induce balanced (Th1 and Th2) humoral responses and potent cellular immunity, which demonstrated an ability to grant protection against pathogens and anti-tumor efficacy in preclinical settings.
mRNA possesses several additional characteristics that makes it a promising tool in the pursuit of a successful vaccine platform. As a synthetic format, mRNA vaccine production avoids the complications of cell- or egg-based vaccine production, requiring only a relevant nucleic acid sequence that can be made accessible safely and rapidly. Unlike plasmid DNA, mRNA poses no danger of genomic integration and there remain no selective markers, such as antibiotic resistance genes, in the final vaccine product. Further, unlike recombinant viruses, mRNA is a minimal, non-replicating, and fully defined genetic vector.
Lastly, it has been demonstrated that mRNA-based vaccines do not require a cold chain during transport and storage, while lyophilized mRNA vaccines have been shown to be stable under extreme temperatures. In the case of prophylactic vaccines, particularly in the developing world, thermostability and product activity under various storage and transport conditions can be the difference between life and death. Extensive testing of mRNA storage has shown good stability. An adequately formulated mRNA vaccine can remain stable for up to 24 months at ambient temperature, and for shorter periods as storage temperatures rise (at 40°C/140°F) mRNA vaccines can remain active for at least six months. Therefore, this novel technology may enable the transportation of life-saving vaccines to people worldwide in safe and affordable ways.
With the first mRNA therapies advancing in the clinic and demonstrating safety, physiologic activity, and a potential for efficacy in patients, the path forward is bright and will expand to include more products in a range of indications. Leading the way will be both therapeutic and prophylactic vaccines intended to activate the immune system. Additionally, mRNA therapies have been developed that enable the delivery of antibodies, enzymes, and hormones without eliciting an immune reaction; while RNA molecules are being created to act as adjuvants to amplify the response to immunotherapies and preventive vaccines.
Key to appreciating the potential of mRNA is understanding that every protein in the world can be encoded by RNA. Given this, mRNA stands out as a truly “disruptive technology” in that it is an active substance administered to the body that is not functional in itself, but provides the information needed by the body to manufacture its own medication. In other words, mRNA is a disease-eradicating platform that transforms each person’s body into its own therapeutic or vaccine factory. Even in this age of recombinant proteins, this idea is truly revolutionary and has the potential to transform medicine as we know it.
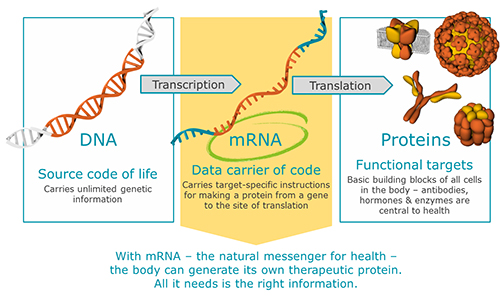
With mRNA – the natural messenger for health – the body can generate its own therapeutic protein. All it needs is the right information.
Ingmar Hoerr, Ph.D., MBA, is chairman and CEO of CureVac.
Reference
1 Kübler H, Scheel B, Gnad-Bogt U, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: A first-in man phase I/IIa study. J Immunother Cancer 2015; doi:10.1186/s40425-015-0068-y







