Sponsored content brought to you by

More than 20 years ago, Michael Chambers and John Ballantyne founded Fargo-based Aldevron to manufacture DNA plasmids for research applications. Over its first two decades the company realized continual growth, acquiring Freiberg-based Genovac in in 2004 for antibody development, and opening a Madison, WI, site for protein development in 2009.
Today, Aldevron is the world’s leading manufacturer of plasmid DNA with nearly 250 employees, and it serves the biotechnology industry with custom production of nucleic acids, proteins, and antibodies. Thousands of organizations use Aldevron-produced plasmids, mRNA, and gene-editing enzymes for projects ranging from basic research to clinical trials to commercial applications.
Expanding Capacity and Capability
Positive advancements in cell and gene therapy are fueling demand for plasmid DNA. In September 2018, Aldevron opened a new 70,000-square-foot manufacturing facility in Fargo, ND, the largest worldwide for plasmid DNA production, to ramp up capacity and to add capabilities for CRISPR nucleases and enzymatically synthesized mRNA, a new product line.
Built from the ground up to meet the company’s specific needs, the facility houses state-of-the-art equipment, HVAC systems, and clean rooms superior to any other existing facility. Infrastructure includes a 300-L single-use fermentation system that increases culturing capacity 10-fold and ramped-up downstream processing equipment to yield larger lot sizes. Plenty of room remains on the 7-acre campus for additional growth.
In March 2019, another expansion added approximately 4300 square feet of laboratory and production space to the Freiberg facility. The space provides immediate room for growth in personnel and equipment for increased cell culture, fusion, production, and sequencing capacity while also reserving space for new services to be launched later in the year.
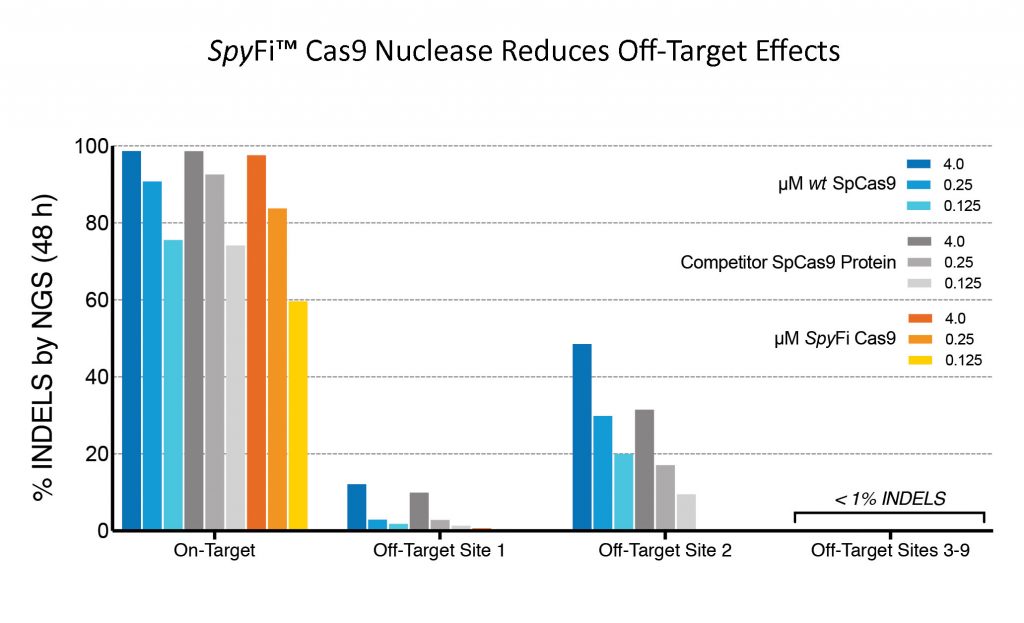 Aldevron SpyFi™ Cas9 has reduced off-target effects. SpyFi Cas9 has measurably lower frequency of off-target editing compared to wt SpCas9. Aldevron’s SpyFi Cas9 Nuclease is sold under license of patents and/or patents pending from Integrated DNA Technologies (IDT). Data shown above was generated in collaboration with IDT.
Aldevron SpyFi™ Cas9 has reduced off-target effects. SpyFi Cas9 has measurably lower frequency of off-target editing compared to wt SpCas9. Aldevron’s SpyFi Cas9 Nuclease is sold under license of patents and/or patents pending from Integrated DNA Technologies (IDT). Data shown above was generated in collaboration with IDT.
Facilitating Science as Products Progress to the Clinic
Aldevron plays a vital role in the value chain of therapeutic research development and supplies three product grades, research, GMP-Source™, and cGMP.
Following strict manufacturing and QC protocols, research-grade products are produced in a nonsegregated environment and released with a certificate of analysis. The next grade level, GMP-Source, is unique and retains many of the hallmarks of a full cGMP batch.
GMP-Source manufacturing occurs in controlled, segregated, non-ISO-classified manufacturing suites; QA reviews critical parameters and processes for documentation and complete traceability. A CAPA system (corrective action/preventative action) operates similar to cGMP-grade protocols, and registered vendors provide the raw materials.
cGMP manufacturing takes place in controlled segregated, ISO-classified manufacturing suites with QA review of all documentation, and full change control and validation. A complete master batch record accompanies product delivery. For custom batches, an optional cGMP-compliant storage facility is available.
Developing Better Inputs for CRISPR
Supplying the best reagents to advance science is part of Aldevron’s culture, and it led to a strategic relationship with Integrated DNA Technologies (IDT) to manufacture a patented, modified Cas9 nuclease.
IDT, in collaboration with Stanford University, Stanford University School of Medicine, Rice University, and Aarhus University, reported in Nature Medicine (Vakulskas et al., 2018), that this new high-fidelity version of Cas9 maintained on-target activity and decreased off-target activity by >10-fold in clinically relevant cell types using clinically-relevant ribonucleoprotein (RNP) delivery methods.
The primary interests of the laboratory group of one of the paper’s contributors, Matthew Porteus, MD, PhD, Associate Professor, Department of Pediatrics and Institute of Stem Cell Biology and Regenerative Medicine, Stanford University, is to develop homologous recombination (HR) as a method of gene therapy for genetic blood disorders, such as sickle-cell disease and b-thalassemia, and primary immunodeficiencies including severe combined immunodeficiency (SCID) and hemophilia.
HR uses an undamaged homologous piece of DNA as a template to repair the break initiated by the Cas9 nuclease. By providing an appropriately designed donor DNA, precise single-nucleotide changes to insertion of large gene cassettes can be made to the genome.
There are different methods to deliver the Cas9 nuclease and gRNA: DNA expression cassettes in plasmids or viral vectors; purified RNAs with Cas9 mRNA; and Cas9-gRNA RNP complexes. RNP delivery results in an initial high level of the genome-editing machinery followed by rapid decay that results in highly efficient editing while minimizing off-target effects.
The drive to maximize safety of genome editing calls for improved versions of Cas9 (and related nucleases) that maintain on-target activity while decreasing potentially harmful, off-target activity, especially those that may lead to major clinically adverse events. Unfortunately, many engineered Cas9 proteins with diminished off-target effects have limited clinical utility because they have significantly lower on-target activity, most likely due to over engineering.
In the Nature Medicine publication, the authors demonstrated clinical utility of the new high-fidelity version of Cas9. It targeted several important disease-associated loci for HR in clinically relevant primary human CD34+ HSPCs (hematopoietic stem/progenitor cells) and T cells. Also, it provided robust correction of the sickle-cell disease–causing Glu6Val mutation in HSPCs while reducing off-target effects up to 20-fold compared to wild-type (wt) Cas9.
This new high-fidelity Cas9 variant is marketed under Aldevron’s trade name, SpyFi™ Cas9 Nuclease. This variant of the wt Streptococcus pyogenes Cas9 sequence contains a single amino acid change from an arginine to an alanine at position 691, and retains 80–100% activity of wt.
Providing the Basis for Breakthroughs
The partnership with IDT gives Aldevron the right to supply, off-the-shelf, research-, GMP-source-, and GMP-grade SpyFi Cas9 Nuclease for research, clinical, and commercial applications.
As demonstrated in the Nature Medicine article, this high-fidelity Cas9 Nuclease functions well in RNP delivery format, and is compatible with ex vivo gene-editing protocols. Inventoried GMP-grade SpyFi Cas9 Nuclease comes with a documentation package to support regulatory filings, 13 validated QC assays, and ongoing stability data.
The release of GMP SpyFi Cas9 Nuclease, along with wt SpCas9 Nuclease, provides researchers a consistent product and continuity from discovery through clinical and commercial manufacturing for gene-editing programs.
“Smaller packs of cGMP-grade SpCas9 and SpyFi Cas9 Nucleases help customers be cost effective when they are initiating Phase 1 trials with low enrollment. SpCas9 comes in 10- and 50-mg sizes, and SpyFi Cas9 comes in 1- and 10-mg sizes. This provides a cost-effective small amount of material for efficacy and safety trials before transitioning to larger volumes for later trials,” explains Tom Foti, Vice President and General Manager, Aldevron.
The ability to purchase GMP-grade prepacked inventoried CRISPR nucleases is a breakthrough, especially for scientists at the tip of the spear of innovation, translating cures from basic research to the clinic, who may not be highly funded.
Gene-editing applications and needs vary. Aldevron also offers AsCpf1 (Cas12a) Nuclease and custom manufacturing of other CRISPR-associated nucleases, including dCas9 (dead Cas9), fusions, base editors, and nickases. Manufacturing scale ranges from 10-mg to multigram lots. Custom nucleases are available at all quality grades.
“Every day we are driven by a passion to enable scientists to make therapeutic breakthroughs that in the end will help patients. Expanding our facility and increasing capacity and capability allows us to facilitate more cutting-edge, translationally relevant science,” adds Foti.
Reference
Vakulskas CA et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nature Medicine 2018; 24: 1216–1224.



