February 1, 2012 (Vol. 32, No. 3)
Sue Pearson Ph.D. Freelance Writer GEN
Over the past decade, the genomics revolution has encouraged scientists to perform target-based drug discovery.
However, among new first-in-class drugs that have emerged in recent years, over 60% have come from phenotypic screening using cell-based assays.
This was a critical and important point made by Steve Ludbrook, Ph.D., cellular section head at GlaxoSmithKline, at the recent SMi “Cell-based Assays” conference.
“As a result, it makes sense to look at some of the more complex assays for our screening projects,” he said.
Peter Simpson, Ph.D., associate director, assay sciences group at AstraZeneca, added, “Many of the current cell assays we’re using are not mimicking the diseases we’re targeting closely enough. For example, if you’re using monolayer CHO cells grown in fetal calf serum, then they’ll behave differently to human cells growing in a tumor.”
Simon Barry, Ph.D., associate director of the oncology iMED at AstraZeneca, agreed.
“In oncology, 3-D cell culture or co-culture assays are very useful in helping us to understand the biology of targets or effects of drugs in more detail. However, we can’t use this information alone. To increase success, we need to think more about the patient as a whole,” he noted.
“In addition to tumor biology, learning more about the effects of a drug on the patient using accessible clinical biomarkers, as for example with serum chemokine/cytokine profiles, would help us use our drugs more effectively.”
According to Stefan Przyborski, Ph.D., founder and CSO of Reinnervate, a spinout company from U.K.-based Durham University, complex cell cultures offer more physiologically relevant models than conventional 2-D cell culture for some applications.
“2-D cell culture limits cell-cell interactions, as up to 50 percent of the cell surface is against plastic and most of the other 50 percent is against the media, so cell signaling mechanisms between adjacent cells are constrained,” he explained.
“Also, if you grow cells in a monolayer, the cytoskeleton remodels. This can have significant effects on the nucleus and, in turn, gene transcription and protein translation, so cells behave in a significantly different manner. By growing cells in 3-D scaffolds, you can get complex interactions between cells and it reduces the stress on cells of being cultured in a monolayer.”
He cited the example of skin models and showed that it is difficult to achieve a stratum corneum layer with existing epidermal models of skin.
“Most skin models are simple and only consist of the epidermis with limited formation of the stratum corneum layer, which is an important component of the skin if you want to test barrier function and drug penetration,” said Dr. Przyborski.
Primary Cells—A Good Starting Point?
Complex cell assays show promise in a number of areas. “Using primary cells such as whole blood cells can reduce the cycle time for lead optimization,” noted Nathan Bays, Ph.D., research fellow at Merck. “They can be used to redeploy existing leads, as the assays are more predictive for disease modification and can provide earlier pharmacological validation for targets identified using genetic/genomic data.”
As an example, Dr. Bays described a screening campaign to determine which compounds used previously to treat cancer could be redeployed to treat inflammatory diseases such as asthma and rheumatoid arthritis. Screening was performed directly in human whole blood, and assays measured changes in protein phosphorylation as well as markers of immune cell activation.
Dr. Bays reported that flow cytometers are in constant use at Merck’s Boston-based research facility, with applications ranging from biomarker discovery to weekly compound titration assays and library screening.
“Using flow cytometry we can measure multiple antigens simultaneously, and a key advantage is we can identify and purify specific cell types,” explained Dr. Bays.
“We now have in place robust automation for safe handling of whole blood, which could be translated to other assays. We are seeing a significant return on our investment because we’re able not only to provide key SAR data using primary immune cells, but we have the ability to perform very focused pharmacological target validation and revise our inhibitor profiles using solid data from disease-relevant cells.”
GSK’s Dr. Ludbrook also spoke about an interesting application of primary cells for drug screening. His group is using frozen primary PBMCs (peripheral blood mononuclear cells) taken from multiple donors to look for IFN-γ inhibition in modified ELISA screens, the Meso Scale Discovery platform, and the proximity ligation assay (PLA).
“We like PLA because it uses the power of qPCR to improve the sensitivity of an ELISA assay. The workflow is not too challenging and we can screen in 384-well plates using automation,” he said.
“The added sensitivity of PLA may enable the screening of rare cells and analytes using this method. To date we have screened one million compounds using the Meso Scale Discovery assay and have found selective leads that inhibit IFN-γ and are potentially useful for disease areas such as colitis.”

The BD Accuri® C6, a 6-parameter personal flow cytometer, is used by scientists at Merck for a range of applications, including biomarker discovery, compound titration assays, and library screening.
The Power of Co-Cultures
According to AstraZeneca’s Dr. Barry, there can be a high stromal fibroblast content in colorectal and lung tumors, which can play a significant role in drug resistance.
“If we model our tumor cells with fibroblasts, this will allow cross-talk and help to develop more realistic platforms to assay compounds,” said Dr. Barry, whose group showed that PK1D tumor cells grown on fibroblasts in co-culture formed a ball and the proliferation pattern of the cells changed.
Using a PLA, he also showed the MAPK/ERK kinase (MEK) inhibitors and drug sensitivity profiles changed.
“In this co-culture environment my dose curves alter by around five- to tenfold and this is perhaps more accurately modeling what is happening in a real tumor,” summarized Dr. Barry. “In fact, for tumors, I believe we should be looking at utilizing these co-culture systems before we take compounds into animal studies.”
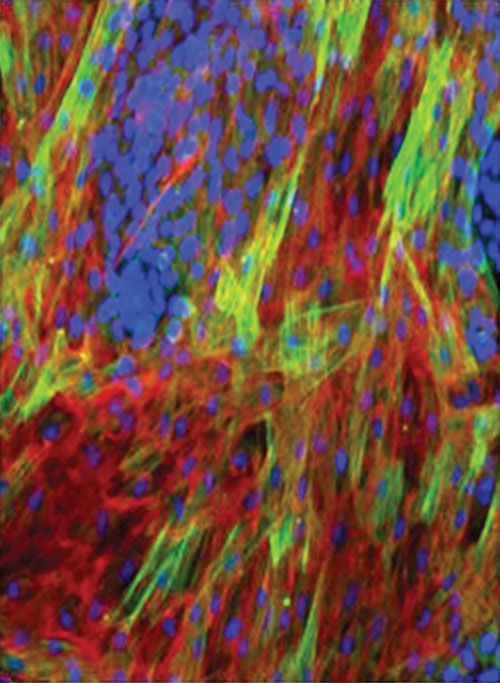
Tumor cell fibroblast co-culture showing tumor cell induction of a-SMA. Tumor cells are stained blue. The activated fibroblasts are stained green. Fibronectin is stained red. Lung tumor cells are shown co-cultured with normal human dermal fibroblasts. [AstraZeneca]
Ultimate Challenge
One reason 3-D cell culture is not yet widely adopted is that there are very few 3-D scaffold options that have been successfully commercialized, noted Dr. Przyborski from Reinnervate. He discussed the use of alvetex®, a polystyrene scaffold of interconnected voids in which cells can enter and grow to form complex 3-D structures.
Dr. Przyborski presented data on hepatocyte cells grown in alvetex that showed that the cells were forming bile canaliculi, producing albumin, and also had elevated levels of a cytochrome p450—Cyp3A4—demonstrating that in the 3-D culture the cells had functionality that more closely mimicked real liver tissue.
After discussing data that showed that skin cultured in alvetex formed keratinocytes and cornified envelopes indicating the formation of a stratum corneum, he also pointed out that human pluripotent stem cells cultured in alvetex formed neuronal rosettes and 3-D neural networks.
“Alvetex is available as well inserts for 12- and 6-well plates, as well as deep-well plates,” said Dr. Przyborski. “Petri dish formats and a 96-well inset will be available in 2012. Scientists can easily reconstruct the 3-D environment for cells with alvetex and it will make 3-D culture as easy and routine to perform as traditional cell culture.”
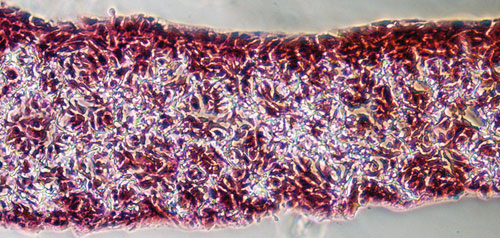
Histological image of the HaCat human keratinocyte cell line growing in 3-D throughout the alvetex scaffold. The culture was fixed, wax embedded, and sectioned in the transverse plane and subsequently counter-stained (H&E). [Reinnervate]
Technology Gap
The speakers all cited either throughput or cost as barriers to the use of complex cell assays for routine screening. As Dr. Ludbrook noted, “At GSK we like cost-effective, fast assays. Some of the more complex cell-based assays using techniques such as flow cytometry are just too low throughput.
“We use flow cytometry in a kinase program to support lead optimization but can only characterize a fraction of the number of compounds we need to. This is why we’re investigating using the HyperCyt® technology for flow cytometry, as this will take us into real-world screening throughput because it reduces plate read time from 120 minutes to 14 minutes for a typical assay, and therefore gives us the potential to screen thousands of compounds in a disease relevant assay.”
“Primary cell assays are expensive,” added Dr. Bays. “With whole blood, you can only get so much blood to work with from one draw and it requires special handling conditions. We use instruments like the Labcyte ECHO to reduce volumes as much as possible, but it still currently costs us about $900 per run for antibodies alone.
“We have not yet reached our goal of spending less than $1 per well. However, we have reduced costs significantly and are not spending anywhere near the $23 per well cost we incurred when we were first developing these assays and had to rely on standard reagent sizes.”
According to Dr. Barry, qPCR is too expensive at the moment for the PLA “but as qPCR and microfluidics are on the point of explosion, it may get to the cost point where we may in the future be able to use this technology for transcriptional screening.”
Dr. Ludbrook identified a wider scientific gap in the understanding of toxicity readouts compared to efficacy readouts.
“The battery of assays used in understanding toxic characteristics of compounds should lead to a reduction in attrition at this stage of drug discovery,” he said.
“However, there is less understanding of translational complex cellular assays that are truly representative of disease. The risk is we could be taking a larger number of safer candidates into expensive and time-consuming Phase II/III trials.
“But if we fail to improve our predictions of efficacy, then we will be failing later and more expensively. We need to focus on this to enable us to bring more medicines to patients at a lower cost of development.”







