Michael J. McClure Ph.D. Virginia Commonwealth University
David J. Cohen M.D.
Allison N. Ramey Virginia Commonwealth University
Caroline B. Bivens Virginia Commonwealth University
Satya Mallu M.D. Virginia Commonwealth University
Jonathan E. Isaacs M.D. Virginia Commonwealth University
Emily Imming Ph.D. Musculoskeletal Transplant Foundation
Yen-Chen Huang Ph.D. Musculoskeletal Transplant Foundation
MoonHae Sunwoo Ph.D. Musculoskeletal Transplant Foundation
Zvi Schwart D.M.D., Ph.D. Virginia Commonwealth University
Enhanced Regeneration Observed in Large Volumetric Muscle Defects
Polytraumatic injuries to the extremities affect soft and hard tissues and can result in permanent loss of skeletal muscle mass, termed volumetric muscle loss (VML).1 These injuries are debilitating and require years of physical rehabilitation,2,3placing a financial burden on the United States of $800 billion annually.4,5 Treatments for VML include pedicle and free muscle transfers or stem cell injections, but they are not effective procedures to restore muscle function and can require additional surgeries and tissue harvest.6
Extensive research has been done to identify more effective VML treatments using animal models with severe functional deficits. In these models, VML typically exceeds 20% of the affected muscle mass and results in reduced muscle function. Wounds this large are far beyond the natural healing capacity, making them a gold standard for regenerative medicine research.7,8 Recent work has recreated VML in mice or rats by partial removal of tibialis anterior,8–13 latissimus dorsi,14,15 or quadriceps.16,17 This replicates the physiological conditions of VML more accurately than other models that chemically induce muscle damage with cardiotoxins.8,18
Extracellular matrix (ECM) structure and chemistry are key elements involved in muscle regeneration and taking advantage of those elements is important to restore function in VML injuries. Muscle ECM is a matrix rich in laminin, fibronectin, collagens, proteoglycans, and growth factors, which play a role in myoblast differentiation and muscle fiber formation.19–28Biomaterials derived from soft tissues can retain these ECM components and have already shown promise.29,30 Several decellularized allogenic and xenogenic matrices are currently available for clinical use, but are exclusively produced from thin tissues such as the skin, small intestine submucosa (SIS), and bladder.31 Those thin-walled tissues do not possess specific properties found in skeletal muscle such as alignment and muscle-specific chemistry. While bladder and SIS primarily contain laminin α332 and α5,33 respectively, muscle contains laminin α1 and α2,34–40 which promote myogenesis in vitro and in vivo.41
Decellularized muscle matrices (DMMs) retain the native morphology of muscle ECM, support muscle healing,19 and promote a proregenerative immune response.41 These matrices release factors in vivo that promote constructive remodeling of tissue by macrophages and suppress a cytotoxic T cell response, resulting in implant integration and tissue regeneration.42 Properties like these are critical to elicit a regenerative response that activates muscle progenitors (satellite cells and myoblasts) to differentiate into myocytes, and fuse together to form muscle fibers.43 Without ECM cues to direct muscle progenitors, muscle healing is delayed.44
In this study, we examined the ability of an allogenically sourced DMM that retains physical and chemical cues to repair a medium 1 × 1 cm and a large 1.5 × 1 cm defect in the rat gastrocnemius similar to studies performed by Merritt et al.45,46Muscle regeneration was examined by investigating muscle function and animal gait, and by histomorphometry and immunohistochemistry of the treated tissues. We hypothesized that DMM would restore muscle function and improve regeneration more effectively when compared to muscle flap (autograft) or type I collagen matrix.
Materials and Methods
Decellularized muscle preparation
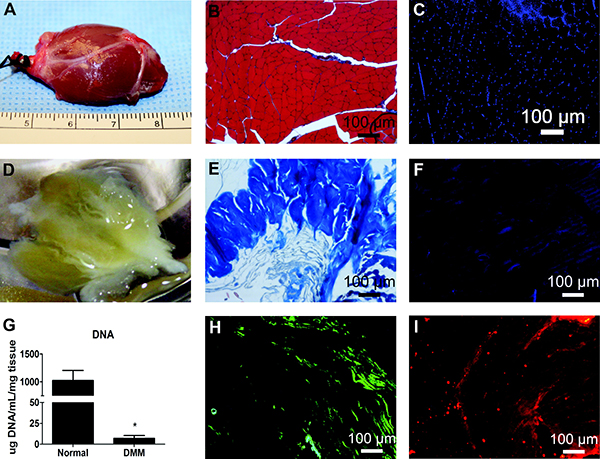
Male Sprague Dawley rats weighing 250–300 g (Harlan Laboratories, Frederick, MD) were euthanized using CO2. Gastrocnemius muscles were isolated bilaterally (Figure 1A), frozen at −80°C, and shipped to the Musculoskeletal Transplant Foundation (MTF, Edison, NJ) to be decellularized. Decellularization was performed by a proprietary method using multiple saline, detergent, and disinfection soaks developed by MTF. The rat tissue was processed aseptically and without any terminal sterilization. Frozen DMMs were shipped back to Virginia Commonwealth University (VCU) and were kept frozen at −80°C until surgery.
Initial experiments validated effectiveness of the decellularization protocols by examining gross morphology and histological structure, and by assessing nuclear staining and DNA content. Gross morphology for whole and decellularized muscle was determined using a Nikon high definition Rebel T3 camera. Paraffin-embedded sections of whole muscle samples were compared to decellularized muscle using Masson's trichrome stain. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (R37606; Invitrogen, Carlsbad, CA) to determine if decellularization resulted in the ablation of cell nuclei. Removal of DNA was confirmed by extracting minced muscle and DMM with 0.1% Triton X-100 and measuring DNA content of the extract using a QuantiFluor dsDNA system (Promega, Madison, WI). The presence of fibronectin and laminin in decellularized matrices was assessed using primary antibodies against laminin-α2 (ab55409; Abcam, Cambridge, United Kingdom) and fibronectin (ab23750; Abcam). Fast Fourier transform (FFT) was used to determine anisotropy. This analytical method was previously shown to effectively qualify fiber alignment in electrospun scaffolds [56].
VML surgery
The literature defines a VML model as one that results in a functional deficit of 20% or greater.10 Based on total muscle size (2 × 3 cm), we determined that a 1 × 1 cm defect in 250–300 g male Sprague Dawley rats would meet this criterion.
Male Sprague Dawley rats (250–300 g) were obtained from Harlan Laboratories and were divided into four groups (n = 8 rats/group): sham, autograft, DMM, and type I collagen plug. All surgical procedures were performed under an approved protocol at VCU. Rats were anesthetized using 4% isoflurane/400 mL/min O2. The surgical area was shaved with clippers and cleaned using 3× alternating swabs of isopropanol and chlorhexidine. Rats were transferred to the operating table, and anesthesia was continued at 1–3% isoflurane in O2. An oblique anterolateral incision extending from the patella to the calcaneus was made, and scissors were used to insinuate deep to the common calcaneal tendon separate it from the calcaneal tuber. After an incision in the biceps femoris muscle to expose the gastrocnemius was made, the left lateral gastrocnemius muscle was carefully separated posteriorly off the superficial flexor muscle toward the gastrocnemius origin in the lateral condyle. A 1 × 1 cm defect was cut in the lateral gastrocnemius, taking care to preserve the tibial nerve (S1A). At the end of surgery, biceps femoris was sutured closed using 5–0 nylon and skin was stapled closed thereafter.
Sham surgeries were performed as described, except when the left lateral gastrocnemius was exposed, no incision was made to create a defect. Instead, biceps femoris and skin were closed without a defect. Rats that received a graft were treated with autograft, DMM, or collagen plug. Autografts were taken from the same gastrocnemius tissue that was removed. These muscle tissue grafts were completely excised from the muscle and then sutured back in place in the same orientation. DMM grafts were sutured into muscle defects taking care to orient the anisotropic features in the direction of the muscle fibers. Collagen plugs were purchased (Resorbable Collagen PLUG; ACE Surgical Supply, Inc., Brockton, MA) and sutured into the defect dry. All sham, autograft, collagen, and DMM rats survived and were harvested at 8 weeks.
To make a more challenging defect, 1.5 × 1 cm defect surgeries were created in 250–300 g male Sprague Dawley rats. In this study, DMM (n = 8) and collagen (n = 8) were used to compare the effect of two different ECM materials that are largely composed of collagen. Sham-operated animals (n = 8) were used as controls. All animals survived surgery and were euthanized at 8 weeks.
Muscle morphology and weight
Immediately following muscle force tests and euthanasia, each gastrocnemius was dissected from the animal with Achilles tendon intact and fat and skin removed. A Nikon high definition Rebel T3 camera was used to take pictures of the treated muscle, which was compared to the contralateral control muscle. Once images were taken with the camera, muscle weight (g) and width (cm) were measured. Following these measurements, muscles were fixed in 4% paraformaldehyde for histological staining.
Histology
Whole gastrocnemius muscles were removed from operated and contralateral legs and fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Muscles were cross-sectioned ∼0.5 cm from the margins. Sections (5 μm) were placed on Histobond slides (VWR, Radnor, PA), deparaffinized and rehydrated, and stained with Harris' hematoxylin (VWR) and eosin-Y (Thermo Scientific, Waltham, MA) (H&E). Sections were also stained with Masson's trichrome using Weigert's hematoxylin (Sigma-Aldrich, St. Louis, MO), Biebrich scarlet-acid fuschin (Sigma-Aldrich), and aniline blue (Sigma-Aldrich). Coverslips were mounted with xylene-based mounting media and allowed to dry flat before imaging.
Histomorphometry
Histomorphometry was used to quantify the percent of centrally located nuclei, myofiber diameter, ratio of muscle to collagen, and average blood vessel density present. Histological sections stained with H&E or Masson's trichrome were imaged using a 10× and 40× objective. Healthy muscle from sham-operated animals was used as a positive control. Healthy muscle fibers were defined as having a polygonal shape with peripheral nuclei and homogenous fiber size distribution. Histological analysis was performed on each muscle (n = 8) in three different locations, taking care to measure only in the graft area (graft margins were not considered in our analysis). Regenerating muscle was defined as myofibers with centrally located nuclei with a small rounded appearance. Myofiber diameter was determined by measuring the shortest distance across the muscle fiber cross-section to avoid the influence of oblique sectioning during data analysis.47 Fibrotic areas were defined as the blue-stained area in Masson's trichrome-stained sections. To assess fibrosis, blue and red color were separated and analyzed using Zeiss imaging analysis software (Zen Blue, Oberkochen, Germany) and a ratio of red to blue was reported as a fibrosis index. Finally, vasculogenesis was determined by identifying and counting each vascular structure (blood vessel) present in each image field. Vascular morphology was defined as rounded structures with identifiable concentric smooth muscle and endothelial layers, or as rounded structures with red blood cells inside the lumen. Average blood vessels present in each image field were reported as mean vascular density. Field sizes of 650 × 870 and 165 × 220 μm were used for histomorphometry.
Immunohistochemistry
Immunohistochemistry was used to stain for fetal myosin heavy chain (fMyHC) paired box 7 (Pax7), nicotinic acetylcholine receptor-epsilon (AChR-?), and nicotinic acetylcholine receptor-gamma (AChR-γ). Two AChR receptors were chosen to distinguish between mature adult AChR-? and fetal receptors (AChR-γ). Sections were deparaffinized and rehydrated before staining. To remove methylated crosslinks from antibody binding sites, slides were incubated at 95°C for 5 min in 10 mM sodium citrate with 0.05% Tween-20. Slides were washed and then incubated with a blocking serum (1.5% goat serum in PBS, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. Samples were washed with PBS/0.6% Tween-20, and incubated for 1 h with primary antibody. Primary antibodies used in this experiment were as follows: mouse anti-Pax7 (ab55494; Abcam); rabbit anti-nicotinic AChR-?, (ab65180; Abcam); mouse anti-nicotinic AChR-γ, (MA3-043; Thermo Fisher Scientific, Waltham, MA); and mouse anti-myosin heavy chain-fetal (fMyHC, SC-53097; Santa Cruz Biotechnology), and were diluted 1:100 in PBS with 1% BSA and 0.3% Tween-20. Samples were washed again with PBS/0.6% Tween-20 and incubated for 1 h with secondary antibody diluted 1:200 in PBS/1% BSA/0.3% Tween-20. Secondary antibodies used in this experiment were from Invitrogen: Alexa Fluor 594 (goat anti-mouse, A11005; goat anti-rabbit, A11012) and Alexa Fluor 488 (goat anti-mouse, A11001; goat anti-rabbit, A11008). Slides were washed thrice for 5 min each with PBS, and then incubated with DAPI (R37606; Invitrogen). Cover slips were mounted with anti-fade mounting media (Invitrogen) and imaged using a Zeiss laser confocal scanning microscope.
To access the the rest of this article and its references click here
Tissue Engineering, published by Mary Ann Liebert, Inc., a peer-reviewed journal, is the preeminent, biomedical journal advancing the field with cutting-edge research and applications on all aspects of tissue growth and regeneration. The above article was first published in the August 2018 issue of Tissue Engineering, Part B with the title "Decellularized Muscle Supports New Muscle Fibers and Improves Function Following Volumetric Injury". The views expressed here are those of the authors and are not necessarily those of Tissue Engineering, Mary Ann Liebert, Inc., publishers, or their affiliates. No endorsement of any entity or technology is implied.