Continuous manufacturing is finally gaining traction in the pharmaceutical industry, but development of the necessary production technologies is, fittingly, a continuous process. Ultimately, the drawn-out development work will deliver worthwhile results.
Using continuous processing instead of batch-based processing gives drug makers several advantages. For example, the continuous approach can accelerate production, cut energy needs, reduce waste, and minimize the risk of human error. The continuous approach is also backed by regulators. The U.S. Food and Drug Administration (FDA) says that switching from batch mode is a “challenging but worthwhile transition.” The European Medicines Agency (EMA) is similarly enthusiastic as part of its advocacy for Quality by Design (QbD).

This support is evident in recent FDA reviews. Since 2015, the agency has approved four products made using continuous processes—two cystic fibrosis drugs (Orkambi and Symdeko, from Vertex Pharmaceuticals), a breast cancer drug (Verzenio, from Eli Lilly and Company), and an HIV drug (Prezista, from Janssen Pharmaceuticals).
Additional approvals are likely, suggests Douglas B. Hausner, PhD, a Rutgers University academic who worked on the Prezista continuous production process. According to Hausner, who is currently affiliated with the Center for Structured Organic Particulate Systems (C-SOPS), the FDA is reviewing more than 30 drugs that are made using continuous manufacturing.
Short-term pressures
Although the pharmaceutical industry is starting to warm to continuous manufacturing, it is undeniable that drug firms have been slower to embrace the approach than compatriots in the oil and chemical sectors. Economic concerns are a major reason for this, says Emil W. Ciurczak, president of Doramaxx Consulting. “Continuous manufacturing is new, and managers are judged on quarterly profits,” he says. “So, change is never viewed as good.” There is, he suggests, always a reluctance to invest, even if an initial outlay of capital can, eventually, save money and result in better products.
Another factor that is slowing adoption of continuous manufacturing relates to how a batch is defined. In drug making, being able to identify the batch to which a drug belongs is important for a variety of reasons. Safety recalls, for example, usually involve withdrawing batches from pharmacy shelves.
Xavier Le Saoût, continuous manufacturing DSP scientific lead at Merck, says, “Quality management is strongly linked with the batch definition, and there is a need for a clear understanding of what qualifies as a ‘batch’ for continuous processing.”
“The issue is really traceability,” asserts Hausner, who adds that “there are solutions [to the problem of batch definition], but it is still new conceptually for organizations. That is the challenge.”
An outdated batch concept
The FDA defines a batch as “a specific quantity of a drug or other material that is intended to have uniform character and quality, within specified limits, and is produced according to a single manufacturing order during the same cycle of manufacture.”
In traditional batch-wise, single-run production, this definition works. However, when manufacturing is continuous and not cyclical, another approach is needed, says Ciurczak. “The FDA and EMA allow a batch to be defined by the company,” he points out. “For example, if you are running the equipment for 24–48 hours, you may define a ‘lot’ as ‘12:00 AM to 12:00 PM, Monday, November 22, 2018’ and continue with the next batch.”
Technological constraints
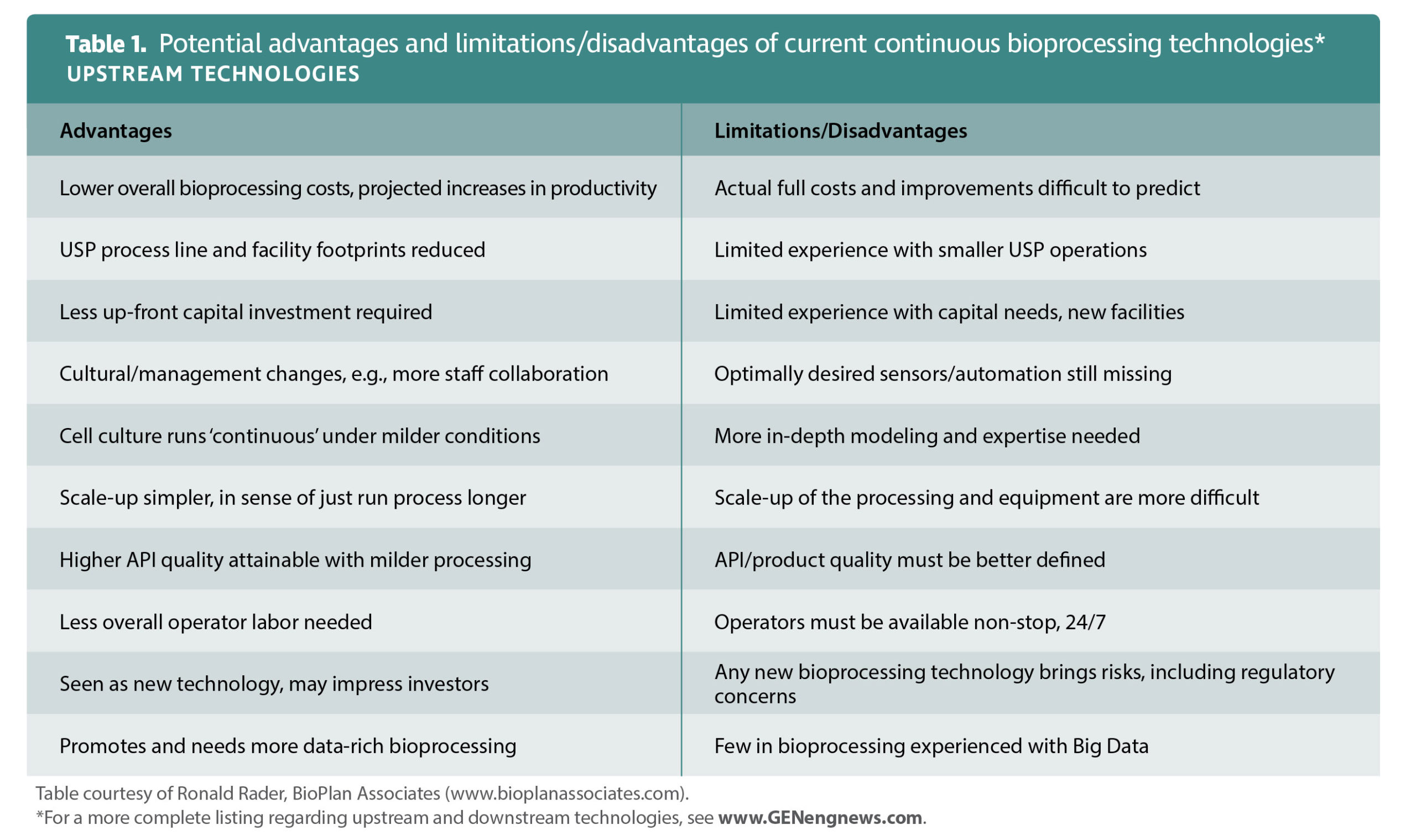
Few technologies are available or widely deployed that can help pharmaceutical companies maintain continuous operations. “On the whole,” Ciurczak declares, “the industry needs all new instrumentation for continuous manufacturing.”
Le Saoût is of a similar opinion. He says that for bona fide continuous manufacturing, new production and monitoring technologies capable of continuous operation are required. “Continuous manufacturing can basically be implemented in two different ways,” he explains. “One way is to design the continuous mode by duplicating each operation, allowing the parallelization of production steps. This solution, which can already deliver important productivity increases, would not require any major innovation in terms of processing technologies as it basically remains a batch operation.”
“Another way,” he continues, “is to focus on achieving a continuous flow. This solution requires a complete change in mindset regarding processing tools.”
Le Saoût emphasizes that process integration and process analytical technology (PAT) are examples of where current technologies need to improve to enable continuous production: “The process control strategy will be modified when comparing continuous flow processing against batch operations. A fully continuous purification process will necessitate extensive PAT tools, which are not commonly used in the batch mode.”
Downstream bottlenecks
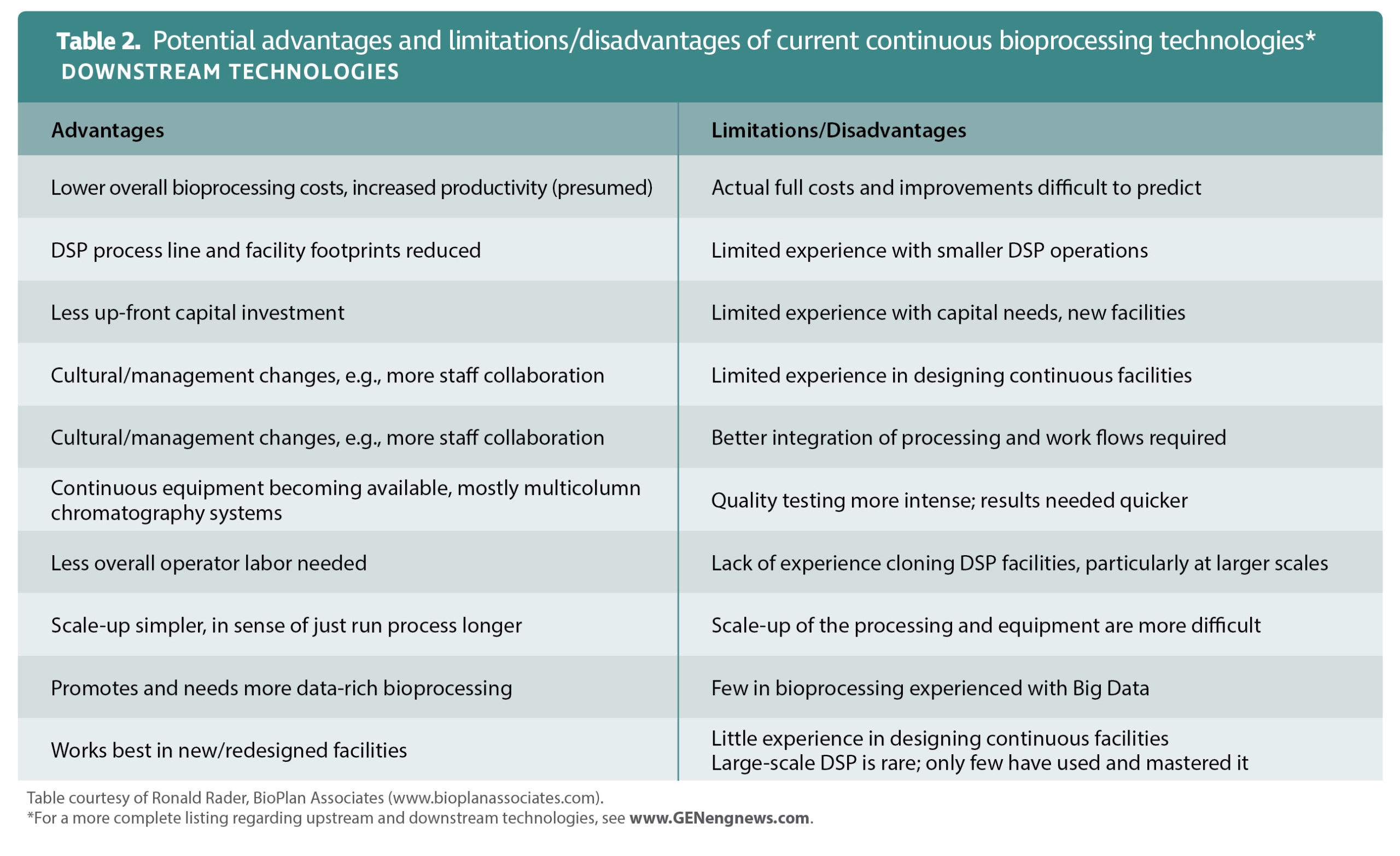
Purification and filtration steps are also more of a challenge in continuous manufacturing than in batch production. This is because few of the technologies used to undertake such operations have unlimited capacity.
“In essence,” Le Saoût says, “every purification step that does not allow complete support regeneration will be much more challenging in continuous purification processes than in batch operations.”
One example of a common downstream operation that is not directly compatible with continuous processing is nanofiltration, a membrane-based method of separating both inorganic and organic substances from a solution. “The only way nanofiltration can be used in continuous mode,” Le Saoût points out, “is to oversize or parallelize, which is strictly opposite to the scope of continuous manufacturing in terms of limiting cost and footprint.”
 Chromatography challenges
Chromatography challenges
Likewise, chromatography is harder in continuous mode than in batch, primarily because it is more difficult to maintain column loading capacity. One way of solving this problem is to use an approach called periodic counter-current (PCC) chromatography, says Lotta Molander, senior global product manager, GE Healthcare Life Sciences.
“The aim of PCC chromatography is to achieve a continuous overloading of the columns or membranes and improve the process efficiency,” she explains. “In PCC, keeping two columns in the loading zone allows for overloading of the first column without the risk of product loss, as the breakthrough will be caught by the second column.” She notes that compared with techniques used in batch operations, PCC offers productivity gains through increased utilization of resin binding capacity. The approach also reduces buffer use.
PCC is particularly suited to large molecule drugs, according to Molander: “Continuous chromatography can be an excellent choice for the capture step when working with sensitive molecules, where continuously removing the molecule from a potentially harmful environment into the first purification step can be the only viable option.”
“In scenarios where there is limited product demand,” she indicates, “continuous chromatography can also allow significant reduction of the needed resin volume through running a higher number of cycles in the available time and thus reducing the column size.”
Real-time monitoring needs
To establish a robust, continuous manufacturing process, a drug maker must have the ability to move materials along the production line seamlessly. Also, it is vital that the drug maker connect the processing systems required for each operation.
“Continuous manufacturing involves bringing a lot of pieces that were handled solo before together,” states Hausner. “Some of these are long-existing tech, others are new. There is less of a tech gap than a know-how gap. There is a need for faster integration of the pieces to save time/cost in getting systems online.”
“Automation is critical because of the complexity that comes with all the pieces coming together,” he stresses. “Automation also unlocks the value of adopting continuous manufacturing.” The technologies used to monitor continuous production must also be able to operate round
the clock.
“Intelligently connecting and integrating several unit operations can support making continuous biomanufacturing economically beneficial through improved process control, predictable product quality, and easier implementation of process analytical technologies,” adds Molander.
“To advance an end-to-end continuous or connected bioprocessing further,” she concludes, “improvements are needed around sensor and process analytical technologies to support enhanced process monitoring and real-time release of the product.”


