Alzheimer’s disease (AD) stakeholders have become increasingly frustrated by the successive failures of developmental drugs in late-stage clinical trials and the failure of any disease-modifying drug based on the amyloid-beta (Aß) hypothesis to attain FDA approval. Despite doubts about its validity, however, the Aß hypothesis is alive and well. Clinical trial failures of Aß pathway-based drugs are understood in the context of right pathway, wrong therapeutic target. Soluble Aβ oligomers (AβOs) have emerged as the right target—the causative agent and most validated and compelling therapeutic target for AD. Two drugs with promising results in Phase II and III clinical trials, BAN2401 and aducanumab, respectively, target AβOs, among other Aβ conformers.
Many drugs that fail in clinical trials because of low or no efficacy are directed against targets in the Aß pathway that do not cause neuronal toxicity and AD, such as Aß plaque, fibrillar Aß, and monomeric Aß. In some cases, drugs demonstrate a low level of activity against AβOs but no efficacy in clinical trials because of off-target distraction. That is, they also attack other, nontoxic targets, leading to ineffective AβO targeting and efficacy failure. The wrong target can also cause safety failures. Drugs that target Aß plaque may cause serious adverse effects (AEs), notably amyloid-related imaging abnormalities-edema (ARIA-E), and fail because of unacceptable AE profiles.
New drug candidates selectively targeting soluble AβOs are now in development and expected to demonstrate greater efficacy and improved AE profiles compared to first-generation Aβ-based drugs. To date, small companies and research institutions are leading the way. Challenges range from the technical (e.g., AβOs are found in small quantities and are difficult to isolate) to business (e.g., high-cost failures have had a discouraging effect on R&D funding of new, novel drugs for AD).
Biopharma industry must pursue soluble AβO-based drug R&D
It has long been clear that very strong scientific evidence supports a causal role for the Aß pathway in AD. There are no plausible alternative hypotheses to the genetic evidence showing that APP, PSEN1, and PSEN2 mutations cause production of Aß plaques and familial Alzheimer’s disease and early-onset Alzheimer’s disease in virtually all carriers. A large body of additional scientific and clinical data supports the role of Aß in sporadic AD.
Discoveries of other factors associated with AD, including viruses, bacteria, metals, medical conditions, and others, are sometimes presented as alternative explanations for the disease. But, rather than contradict the Aß hypothesis, they fit into it as risk factors that may regulate expression of AD risk genes and Aβ processing genes. Any alternative hypothesis of AD pathogenesis would need to account for Aβ pathway activation and the cytotoxicity, pathology, and disease progression findings consistent with the natural history of AD.
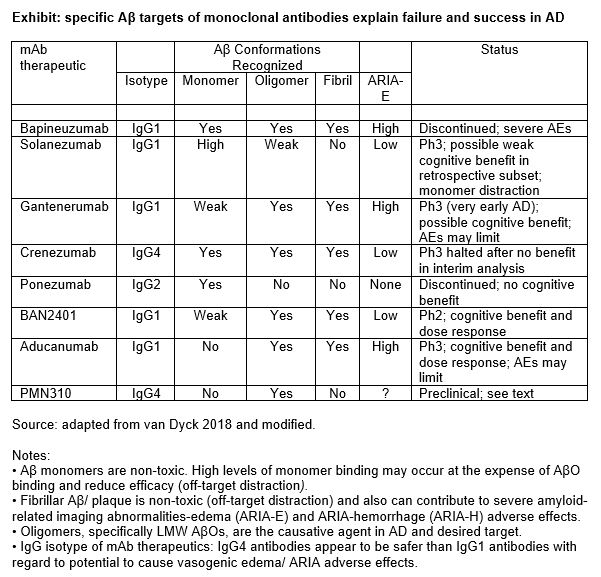
“Which specific Aß pathway product/species is the neurotoxic, causative agent in AD?” was the burning issue in the 1990s after Aß plaque was found to be not toxic (or minimally neurotoxic) and incapable of causing the massive neuronal cell death found in AD. Soluble AβOs were identified as the neurotoxic Aß species and causative agent; and the Aß hypothesis was revised by 2002. The revision had two critically important implications for AD drug developers: targeting Aß plaque would not be effective; and drug R&D must shift to targeting AβOs. It is striking to observe that, more than 15 years after the Aß hypothesis was revised, none of the anti-Aß monoclonal antibody (mAb) drugs tested in Phase II or III clinical trials was designed or optimized to selectively target soluble AβOs. And funding of internal or external R&D programs based on AβO targeting have not been announced by mid-sized or large biopharma companies. Figure 1 shows that Aβ target specificities can predict efficacy and adverse effects.
The absence in late-stage clinical trials of any drug candidate selectively targeting AβOs is explained, in part, by delays caused by initial R&D efforts in pursuit of the wrong target (Aβ plaque) and the years required to pursue new R&D targets from discovery through regulatory approval. The failure to invest in AβO-targeted R&D, however, is the most important factor. As a result, drugs that have reached Phase II or III clinical trials were discovered in the 1990s or early 2000s. None selectively targets AβOs, but aducanumab and BAN2410 demonstrate significant binding to AβO, among other Aβ conformers.
It is the continued failure of biopharma industry to embrace and invest in AβO-based drug R&D programs that is more surprising. Reasons include companies are awaiting completion of Phase III trials, the recent trend to treat very early AD has caused uncertainty in the clinical development paradigm, AβOs have been a technically challenging target, and lack of understanding of mechanisms underlying AβO toxicity. AβOs, formed by Aβ protein misfolding, act as Aβ seeds for propagation of toxic structures via a corruptive protein templating mechanism. This prion-like propagation and spread of toxic AβO is consistent with patterns of insoluble Aβ deposition and the role of soluble AβO as the causative agent in AD. Recent research has proved the transfer of amyloid pathology between humans through proteopathic Aβ seeds present in surgically transferred material. These findings demonstrate how soluble AβOs, which are present in the brain in small quantities, cause Aβ amyloidosis and AD.
PMN310: A highly selective anti-AβO mAb
ProMIS Neurosciences has developed a platform for discovery of highly selective mAbs that target misfolded toxic oligomers. The platform is based on visualization technology for prediction of epitopes (both target sequence and conformation) that are inaccessible by other techniques, followed by generation and characterization of antibodies. Drug candidates are selected based on selectivity of binding profiles and in vitro and in vivo tests of functionality. In AD, the ProMIS platform targeted epitopes displayed on toxic AβOs but not on other forms of Aβ (monomer, plaque, and protofibrils). The company’s lead drug, PMN310, is a mAb with greater selectivity for AβOs than aducanumab (Phase III) and BAN2401 (Phase II), which are the most developmentally advanced mAbs that significantly target AßOs. Neither aducanumab nor BAN2401 was designed or optimized to selectively target AßOs, and both bind to other Aß species.
In preclinical efficacy studies, PMN310 demonstrated greater therapeutic potency than aducanumab and BAN2401, CNS penetrance equal to that reported for aducanumab and no off-target monomer binding (i.e., no potential for off-target distraction). Findings from preclinical safety were positive. PMN310 did not bind to Aß plaque in AD brain tissues, unlike aducanumab and BAN2401, which demonstrated binding to Aß plaque (fibrils) in the brain and blood vessels. These findings suggest the potential for a restricted maximum dose and ARIA-E adverse effects with these two drugs. [In fact, the maximum dose of aducanumab has already been restricted because of the potential for ARIA-E adverse effects.] In addition, PMN310 is based on an IgG4 antibody isotype, which carries no known ARIA-E risk factor. BAN2401 and aducanumab are based on IgG1 antibody isotype, which increases the risk of causing ARIA-E AEs. Based on all comparative studies, to date, PMN310 demonstrates the potential to become the best-in-class, selective AßO-targeted therapeutic for safe and effective treatment of AD.
IND enabling development work is underway to prepare PMN310 for an initial Phase I, ascending dose clinical trial, with initial clinical results reporting by the end of 2020.
Elliot Goldstein, MD, is president and CEO; Neil Cashman, MD, is CSO and co-founder; James W. Kupiec, MD, is chief medical officer; and Johanne Kaplan, PhD, is chief development officer at ProMIS Neurosciences.
Acknowledgement
Timothy Tankosic, MD, managing director, Aqua Partners, provided research and writing assistance.






