Could a mother’s diet and excess weight during pregnancy predispose her offspring to become obese and develop metabolic disorders and cardiovascular diseases later in life? U.S. researchers report on new molecular and genetic studies in baboons that support existing epidemiological evidence suggesting that maternal obesity (MO) may “preprogram” offspring to develop cardiometabolic diseases in adulthood by causing changes in key metabolic pathways in the developing fetal liver. The results showed that baboon fetuses of obese baboon mothers who consume high-sugar, high-fat diets during pregnancy develop a fatty liver and exhibit significant metabolic disruption in the liver, including changes to gene expression profiles and epigenetic modifications.
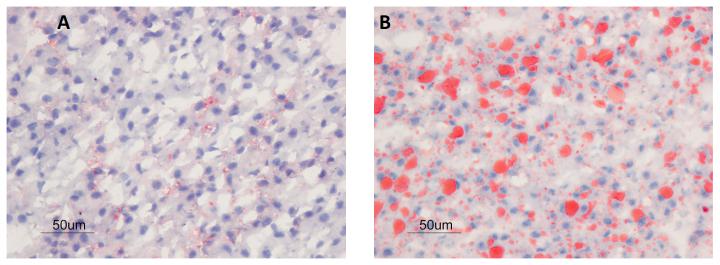
“It wasn't until we saw the microscope slides for the staining of liver sections showing very high amounts of lipid in fetuses of obese mothers that we realized the dramatic impact of maternal obesity at such an early developmental time point,” comments Peter Nathanielsz, M.D., Ph.D., at the University of Wyoming Department of Animal Science, Laramie, WY. “Histological analyses of these livers showing the condition steatosis underlined the detrimental impact of MO on the developing fetus.”
The study results, published in The Journal of Physiology, are reported by the University of Wyoming researchers and collaborators at the Texas Biomedical Research Institute, Wake Forest Baptist Medical Center, Indiana University School of Medicine, and the University of Texas Health Science Center. Their paper is entitled “Primate Fetal Hepatic Responses to Maternal Obesity: Epigenetic Signalling Pathways and Lipid Accumulation.”
Approximately 64% of women of childbearing age in the U.S. are now either overweight or obese, the researchers report. Previous research in multiple species has linked maternal obesity and excess maternal weight with increased risk of obesity in their offspring and demonstrated that MO causes “developmental programming” effects, which predispose offspring to develop cardiometabolic disorders later in life.
“In light of these studies, we hypothesized that MO would induce epigenetic changes in fetal baboon (Papio hamadryas) liver resulting in dysregulation of key metabolic pathways that impact lipid metabolism,” the authors state. They devised a series of studies to evaluate the effects of pre-existing maternal obesity and a high-fat, high-sugar diet during pregnancy in baboons on the developing fetal liver, and in particular on gene expression, resulting microRNA (miRNA) expression, and signaling pathways. “This is the first study where unbiased transcriptome analysis, including gene expression and miRNA expression, has been used to identify potential miRNA epigenetic regulators of metabolic disruption in nonhuman primate fetal MO livers,” the researchers claim.
Their results showed that the livers of fetuses developing in obese mothers accumulated three times as much lipid than control fetuses in nonobese mothers fed on a normal diet. “Our results showing increased lipid accumulation in MO baboon fetal liver suggest that these offspring are on an early trajectory to develop NAFLD [nonalcoholic fatty liver disease] and NASH [nonalcoholic steatohepatitis],” the authors write.
Gene expression profiling showed that maternal obesity also led to altered expression of fetal hepatic genes that are central to metabolism. This included dysregulation of the tricarboxylic acid (TCA) cycle, proteasome, oxidative phosphorylation, glycolysis, and Wnt/ß-catenin signaling pathways. “These well-characterized, inter-connected pathways have been shown to be important for liver metabolic function,” the authors state. The TCA cycle and oxidative phosphorylation are both central to the production of energy in the form of ATP. The proteasome is a multicatalytic enzyme complex, which plays an important role in degradation of both normal and damaged proteins, while the he Wnt/β-catenin signaling pathway plays an important role in development, tissue homeostasis, and oxidative stress, and is involved in the development of hepatic fibrosis and hepatocellular carcinoma.
Quantitative miRNA analysis of fetal livers indicated that altered epigenetic mechanisms may underpin metabolic pathway dysregulation. The analysis initially identified 67 miRNAs that were differentially expressed between the livers of fetuses in obese and nonobese mothers. Further prioritization honed this initial list down to 11 miRNAs targeting 13 genes in the affected pathways. “…fetal hepatic miRNA-gene interactions suggest that MO influences these interconnected pathways regulating cell proliferation, liver steatosis, hepatic fibrosis, and lipid metabolism through epigenetic mechanisms,” the team writes. “These miRNA-gene interactions suggest important early molecular mechanism by which MO programs fetal hepatic lipid metabolism.”
The researchers aim to investigate the metabolic and cardiovascular health of monkey offspring of obese mothers, including liver function, at intervals throughout life, with a view to gaining new insights into whether the consequences of maternal obesity may even pass down to grandchildren. “Further studies are required in MO postnatal offspring to determine the extent to which the fetal phenotype persists, and the degree to which this increases offspring risk of cardiometabolic disorders in later life,” they note.
“This research is important as throughout the world over 50% of women of reproductive age are overweight or obese,” Nathanielsz states. “Maternal obesity, combined with high-fat, high-sugar diets, makes it more likely that children will suffer from liver disease and face health problems such as obesity and heart disease later in life.”