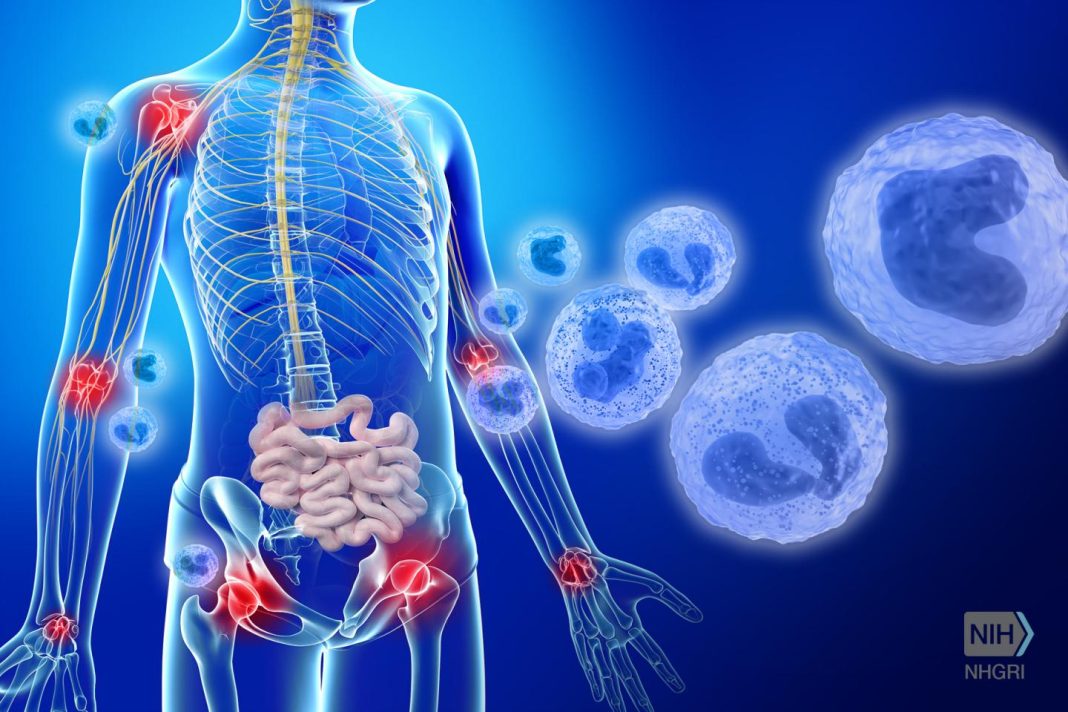
Inflammation has always been a double-edged sword, providing critical action that helps heal wounds or thwart infections, in addition to damaging healthy cells and tissues due to a disproportionate response. Understanding the regulatory mechanisms that control the balance between positive and negative inflammation could open new drug discovery avenues for a variety of diseases and disease complications, such as sepsis. Now, investigators from the University of Illinois at Chicago (UIC) have identified a protein that they believe is critical for activating inflammation. Findings from the new study were published recently in Immunity, in an article entitled “The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation.”
Scientists have long known that inflammation involves the activation of a structure within immune cells called the inflammasome and that it is activated by an influx of potassium ions across the cell membrane through a protein channel. However, the identity of this channel was not known until now. The UIC team is optimistic that the newly identified protein—an ion channel called TWIK2 that spans the membrane of immune cells—presents a new target for the development of drugs that can restrain overblown inflammatory responses.
“Now that we have identified this crucial channel, it opens up the possibility of developing targeted new anti-inflammatory drugs to modify its function and help and reduce inflammation,” explained senior study investigator Asrar Malik, Ph.D., professor and head of pharmacology in the UIC College of Medicine. While some drugs currently exist that target potassium channels, drugs specific to the TWIK2 channel still need to be developed.
Sepsis is a very serious condition and a potentially life-threatening complication due to infection in humans. In a mouse model of sepsis, where the immune system has an overblown response to a bloodstream infection, mice lacking the TWIK2 molecule had significantly reduced levels of inflammation and activation of the inflammasome in their macrophages was suppressed. Moreover, when the researchers transferred macrophages lacking TWIK2 into a mouse model of sepsis where native macrophages were depleted, inflammatory lung injury was prevented.
“Knowing that TWIK2 is the channel that leads to activate the inflammasome allows us to dial down the inflammation response with a drug that inhibits TWIK2 when inflammation gets excessive,” Dr. Malik noted.
Interestingly, the researchers noted that quinine, a bitter crystalline compound present in cinchona bark that has been used since the 18th century as an antimalarial and antifever drug, inhibits the function of the TWIK2 channel in macrophages.
“Some of the fever-suppressing effects of quinine may be due to its effects on the TWIK2 channel,” remarked co-senior study investigator Jalees Rehman, M.D., an associate professor of medicine and pharmacology in the UIC College of Medicine. “We found that quinine reduced the levels of the inflammatory molecule interleukin-1β, which is known to cause fever.”
Unfortunately, quinine and many other anti-inflammatory medications used to treat excessive inflammatory responses have an array of negative side effects. The UIC is hoping that their research will provide new opportunities for improved drug development.
“By discovering new components of the inflammation pathways, we hope to pave the way for new personalized anti-inflammatory drugs which minimize the side effects for patients,” Dr. Rehman concluded.