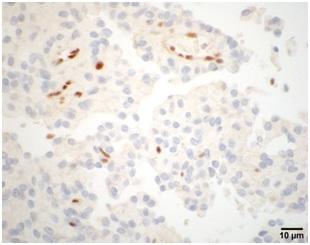
![BAP1 immunohistochemistry of a rhabdoid meningioma sample from a patient that carries a BAP1 mutation. The protein is completely lost in the tumor cells but is still fully maintained in normal blood vessel cells and in infiltrating fibroblasts and immune cells. [Sandro Santagata/Brigham and Women's Hospital]](https://genengnews.com/wp-content/uploads/2018/08/Nov10_2016_BrighamWomenHosp_BAP1Immunohistochemistry1691856121-1.jpg)
BAP1 immunohistochemistry of a rhabdoid meningioma sample from a patient that carries a BAP1 mutation. The protein is completely lost in the tumor cells but is still fully maintained in normal blood vessel cells and in infiltrating fibroblasts and immune cells. [Sandro Santagata/Brigham and Women’s Hospital]
Scientists from Brigham and Women's Hospital (BWH), in collaboration with colleagues at Massachusetts General Hospital, have identified genetic mutations in the form of brain cancer known as meningioma that can distinguish aggressive rhabdoid meningiomas from more benign forms using routine laboratory tests. The new findings could have immediate implications for clinical decision-making, according to the researchers.
Their study (“Germ Line and Somatic BAP1 Mutations in High-Grade Rhabdoid Meningiomas”) is published in Neuro-Oncology.
Meningiomas are the most common primary brain tumors, but the term encompasses over a dozen subtypes that range from benign to highly aggressive. Rhabdoid meningiomas are classified as highly aggressive due to their high rates of recurrence and mortality, but the experience and outcomes for patients with this rare form of brain tumor vary widely.
“Our work has practical implications for the care of patients with meningiomas,” said Sandro Santagata, M.D., Ph.D., a pathologist in the Neuropathology Division at BWH. “We hope that this work will have an impact not only on clinical management, but also for the enrollment of patients in clinical trials. Novel treatments for meningiomas are urgently needed, and determining which patients have more aggressive forms of the disease based on the tumor's genetics could help us design better trials and more precise treatment approaches.”
Ganesh Shankar, M.D., Ph.D., a neurosurgeon at Massachusetts General Hospital, noted that, “Two meningiomas may appear similar under the microscope, but we know these two tumors may have very different clinical courses. The goal of this study was to identify an easy-to-measure feature that would help predict which tumors require closer surveillance and upfront therapies following surgical resection to delay progression. This is an effort that drew on the resources of multiple collaborating institutions working toward the same goal.”
Usually, rhabdoid meningiomas are classified on the basis of physical appearance and characteristics, but these enigmatic tumors can be difficult for pathologists to classify accurately. To find a molecular fingerprint that could help identify rhabdoid meningioma, Dr. Santagata and his colleagues sequenced 560 cancer-related genes from 14 meningiomas. In one sample, the team detected a mutation in the BAP1 gene along with telltale physical features (such as the shape of the tumor cells) of rhabdoid meningioma.
Previous studies had found that people with inherited mutations in the BAP1 gene that cause a loss of BAP1 protein are prone to a tumor predisposition syndrome, a condition that puts them at a very high risk of developing several kinds of tumors, including tumors in the eye, lung, kidney, and elsewhere. But primary brain tumors had not been associated with the syndrome before.
The team went on to analyze samples from 47 patients with rhabdoid meningiomas as well as 265 additional meningiomas of diverse subtypes and grades. None of the nonrhabdoid meningiomas had a loss of BAP1. However, 5 of the 47 patients with rhabdoid meningiomas did have mutations or deletions affecting BAP1. These patients had poor clinical outcomes. Two died of the disease and two had multiple cases of recurrence; clinical follow-up information was not available for the fifth. For those patients with intact BAP1, average time of disease progression was 116 months; for the patients with BAP1 mutations, it was only 26 months.
The presence or absence of BAP1 can be monitored with an immunohistochemistry test in which a tissue sample is collected and stained for a particular protein. This approach is currently in routine use for characterizing samples of an eye cancer known as uveal melanoma and a tumor that arises from the linings of the chest and abdomen known as mesothelioma (forms of cancer tied to the tumor predisposition syndrome).
The number of cases of rhabdoid meningioma studied in this work was small; larger studies will be needed to determine the prevalence of BAP1 mutations in rhabdoid meningiomas and to assess the impact of their detection on clinical care. However, the new work strongly suggests that a careful assessment of family history is critical for patients who develop rhabdoid meningiomas and that patients with BAP1-negative tumors may warrant more careful observation and focused care.
“Testing for BAP1 in rhabdoid meningiomas could be performed routinely and at a low cost, with the potential to change the course of clinical care and avoid overtreatment or to identify those who may need more aggressive therapy,” said Dr. Santagata. “We hope that this new work will offer insights for clinicians and patients alike as they seek more information on these tumors.”