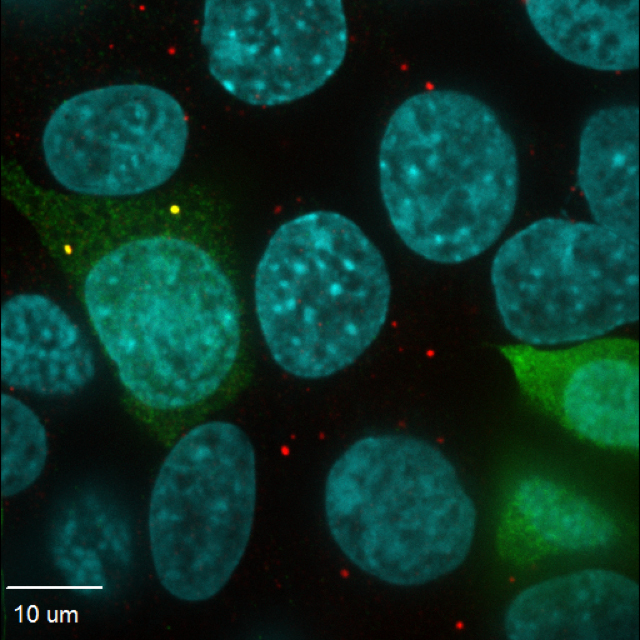
![Human kidney cells stained with a P-body marker (red) and NoBody microprotein (green). Yellow dots are where P-bodies and NoBody interact. Cell nuclei are shown in blue. [MIT/Yale]](https://genengnews.com/wp-content/uploads/2018/08/Dec6_2016_SalkInst_OverlayNuclei1131052919-1.jpg)
Human kidney cells stained with a P-body marker (red) and NoBody microprotein (green). Yellow dots are where P-bodies and NoBody interact. Cell nuclei are shown in blue. [MIT/Yale]
When it comes to used messenger RNA (mRNA), we know NoBody knows where it comes and where it goes. This NoBody, unlike the nobody of Aerosmith fame, may not inspire song, but it helps fulfill a vital function: mRNA recycling.
NoBody, named for nonannotated P-body dissociating polypeptide, is a 7-kDa human microprotein. It is, like other microproteins, so small that it remains unseen unless special techniques are used. Yet it did not escape the notice of Salk Institute and Yale University researchers. They identified NoBody by using a technique that has revealed more than 400 new proteins.
Details of the researchers’ work appeared December 5 in the journal Nature Chemical Biology, in an article entitled, “A Human Microprotein That Interacts with the mRNA Decapping Complex.” The article emphasizes that NoBody is notable not only because it enables a form of genetic housekeeping duties, but also because it encourages us to study other microproteins. NoBody suggests that microproteins may be essential to all manner of biological processes.
“NoBody interacts with mRNA decapping proteins, which remove the 5′ cap from mRNAs to promote 5′-to-3′ decay,” wrote the article’s authors. “Decapping proteins participate in mRNA turnover and nonsense-mediated decay (NMD).”
NoBody interacts with proteins involved in the mRNA recycling process known to form P-body granules—clusters of mRNAs and proteins that perform the first step in breaking down the mRNAs. According to the authors, “Modulation of NoBody levels reveals that its abundance is anticorrelated with cellular P-body numbers and alters the steady-state levels of a cellular NMD substrate,” the authors explained. By highlighting NoBody’s function, the authors have identified a potential target for future therapeutics related to RNA dysfunction.
“The fact that NoBody has been present in this intensively studied complex of proteins all this time, but completely escaped our notice, really hammers home how many more currently unknown microproteins could be associated with essential cellular machineries,” said Sarah Slavoff, Ph.D., one of the current study’s co-authors and an assistant professor of chemistry and of molecular biophysics and biochemistry at Yale.
To get around issues of detection and find out what small microproteins might be overlooked, the paper's authors combined genomic sequencing and protein mass spectrometry (proteomics) to predict and identify nonannotated microproteins. The team began by isolating the contents of cells from the commonly studied myeloid leukemia cell line, removing the larger proteins to leave behind only the smaller ones. Then they used an analytical chemistry technique called liquid chromatography-mass spectroscopy proteomics to determine the amino acid sequences of every protein, including microproteins, that were present in the sample.
To figure out which genes these mapped to, the team used a homemade computational method to predict every possible microprotein from a myeloid cell's total mRNA content, which they sequenced using genomics techniques. This custom database was then used to search their proteomics data for novel microproteins and led to the discovery of over 400 novel microproteins, including NoBody.
“Despite how much we know about the human genome, there are still blind spots in the genome discovery algorithms,” noted Alan Saghatelian, Ph.D., Salk professor and the current study’s other senior author. “You can sequence the whole human genome and never know a protein, like this one, was there because it's too short and falls below the usual length requirement for gene assignment algorithms.”
The members of the Salk/Yale team think that NoBody might signal other important microproteins that are involved in disease. Protein granules are found in many biological processes and are of particular relevance in neurological diseases, where proteins clump and aggregate together, such as in the amyloid plaques that are associated with Alzheimer's disease.
“While NoBody is not directly involved in Alzheimer's disease or other diseases, this discovery suggests that other microproteins might be,” said Saghatelian. “The search for and characterization of these other microproteins in biology and disease represents an exciting frontier in molecular biology.”