Disruptive technologies, as well as novel therapeutic candidates, were presented by many biotechs at the recent BioTrinity conference organized by OBN, the U.K.’s largest biotech networking group. Across the spectrum of antibody, small-molecule, cell, and gene therapies, there were some interesting in-licensing, partnering, and acquisition opportunities on offer.
Not to be Sneezed At
One of the stand-out technologies discussed by London-based immuno-oncology start-up IGEM Therapeutics was in antibody therapeutics, an area where there are often interesting variations on a theme but not much in terms of new platforms available. The firm is the exception to this rule and is developing antibodies to treat a variety of solid tumor-based cancer using antibodies with IgE backbones in place of IgG.
Tim Wilson, Ph.D., CEO at IGEM Therapeutics put forward some sound arguments as to the benefits of using these types of antibodies. Dr. Wilson explains: “IgE antibodies aren’t just there to make you sneeze, they are actually highly adapted to fight parasites such as schistosomes and helminths which live in tissues. This means they have several key features which IgG antibodies don’t, making them ideal for the treatment of solid tumors, which are also to be found in tissues. This is borne out by the research of Sophia Karagiannis, Ph.D., and her team at Kings College London, who showed that IgE antibodies are more effective at destroying tumor cells than IgG equivalents in a range of preclinical cancer models.”
According to Dr Wilson, IgE works well at tumor destruction because the epsilon constant region of IgE binds tightly to FcεRI receptor on the surface of immune effector cells such as macrophages, monocytes, basophils and eosinophils. “IgE antibodies essentially help the troops to knock down the castle walls more effectively,” comments Dr. Wilson.
“This interaction is up to 10,000-fold greater than the gamma chain of IgG has for its receptor, resulting in most IgE molecules being very tightly attached to the surface of immune effector cells.” IgE antibodies are therefore able to permeate tumors more effectively than IgG equivalents and stimulate greater levels of both ADCP (antibody-dependent cell-mediated phagocytosis) and ADCC (antibody-dependent cell-mediated cytotoxicity), the two main mechanisms by which immune-effector cells kill tumor cells.
“Additionally, although IgE antibodies have a short half-life in blood, around six hours, and they also have a half-life of two weeks in tissue whereas IgG antibodies tend to have a short tissue half-life of just two to three days.
“In the early 1990s, there were a number of failures in clinical trials of therapeutic antibodies and most were being used to treat solid tumors residing in tissue. Now, all the main commercial antibody therapeutics are IgG based and the majority are still not that good at dealing with solid tumors so using IgE-based antibodies would be a truly disruptive approach to this problem,” Dr. Wilson concludes.
The company secured a £2 million Series A investment in April 2017 from Epidarex Capital and has accessed a further £1.8M in grant funding. It is developing a portfolio of IgE antibody candidates, including those that target folate receptor alpha, HER2, and epidermal growth factor receptor (EGFR). Their lead candidate, IGEM-F, is currently in a Phase 1/2a trial to treat ovarian cancer and is the world’s first IgE therapeutic to enter the clinic.
Eyes on the Prize
Therapies for treating ophthalmic diseases were also noteworthy at BioTrinity, with Exonate and Eyevensys presenting data on some promising clinical candidates. Exonate, based in Cambridge, U.K., is a spin-out biotech from the University of Nottingham. The firm is collaborating with scientists from the University of New South Wales, Australia, to develop topical eye drops to treat retinal vascular diseases.
According to Catherine Beech, Ph.D., CEO of Exonate, “With an aging population and the increase in type 2 diabetes, the number of people suffering from wet macular degeneration (AMD) is expected to increase by more than 50% by 2020. If people are left untreated, they will lose their sight within two years.
“Unfortunately, the most effective treatment currently is a vascular endothelial growth factor (VEGF)-inhibitor, but this must be injected directly into the eye once a month. Many patients would prefer a less-invasive treatment, so we are developing eye drops containing small molecules which inhibit the production of pro-angiogenic VEGF.”
Exonate’s pipeline of small-molecule therapeutic candidates, known as SPHINXes, inhibit a protein kinase called serine/threonine-protein kinase (SRPK1). This is a key regulator of messenger RNA (mRNA) splicing, which produces different forms of proteins from the same mRNA. During angiogenesis-related diseases such as wet AMD, SRPK1 promotes production of a pathological form of the VEGF protein that drives angiogenesis, so inhibiting this protein could potentially prevent or slow the progression of retinal degeneration and blindness.
Dr. Beech presented data which showed that in preclinical mouse and rabbit models the company’s lead product candidate, designated EXN169, can permeate to the back of the eye and remain there for 16 hours but is not available systemically. She also showed that in preclinical models of wet AMD, EXN169 reduces retinal thickness indicating it may slow down disease progression.
“Our technology has the potential to offer safer and more effective therapies for wet AMD,” says Dr. Beech. “We will continue our preclinical work with primate models this year and hope to move into Phase I studies by 2020. Then, we will offer our technology as licensing and milestone payment deals and we have already had considerable interest from big pharma partners.”

Paris-based biotech Eyevensys is taking a very different approach and is using its EyeCET–Electrotransfection system to deliver a plasmid-based gene therapy rather than viral gene therapy which produces therapeutic proteins in the ciliary muscle of the eye.
“Electrotransfection is minimally invasive, and it takes less than five minutes to deliver a plasmid which will continue to express a protein over time,” explains Patricia Zilliox, Ph.D., CEO at Eyevensys. “The effects of the treatment could last up to six months or more because the therapeutic protein will get into the vitreous humor via the ciliary muscle. This approach means that patients won’t need intravitreal implants or repeated intravitreal injections in their eye.”
The firm’s lead product, EYS606, uses a plasmid encoding for production of an anti-tumor necrosis factor (TNFε,). TNFε, is a pro-inflammatory cytokine that has been shown to enhance intraocular cytotoxic events in uveitis and EYS606 is being developed to treat non-infectious uveitis (NIU).
“Our lead product has shown good durability in preclinical rabbit models and we are already in Phase I-II clinical trial in France—and have to date successfully treated four patients, who have shown no safety issues,” continues DR. Zilliox. “We have also had a successful pre-IND meeting—and are planning to file IND by early 2019 to allow us to begin a Phase II-III study. We expect this product will follow a faster development pathway than many therapies because it has been granted Orphan drug designation by the EMA for the treatment of NIU.”
Preventing Amputation
In the cell-therapy arena, Rexgenero was the one to watch, presenting some promising data on its regenerative therapy. The London-based firm manufactures stem cells at its manufacturing site in Spain and its lead product, an autologous bone marrow-derived mononuclear cell therapy known as REX-001, is in clinical trials to treat some of the serious complications caused by critical limb ischemia (CLI).
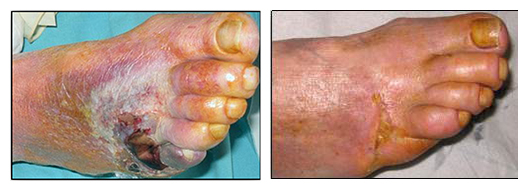
Joe Dupere, Ph.D., CEO at Rexgenero, described the effectiveness of REX-001 in an account of its effects on one of the CLI patient.
“One of our male patients is 66 years old, has type 2 diabetes and CLI. When he came on the clinical trial, he had had a non-healing leg ulcer for six months, was at high risk of gangrene and was likely to have an amputation,” says Dr. Dupere. “Several of the blood vessels in his foot and leg were blocked, he had pain when he walked and couldn’t sleep because of the pain. After the REX-001 treatment his ulcer healed, and his leg has revascularized. Now he can sleep at night, walk and even play football with his grandchildren.”
This patient is not an isolated success, according to Dr. Dupere. In Phase II trials of 60 CLI patients, 12 months after treatment with a single infusion of REX-001, two-thirds showed vascular regrowth and after six months these same patients had complete healing.
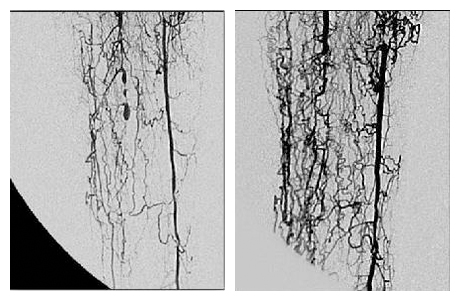
REX-001 is currently being manufactured in an automated process at Rexgenero’s manufacturing facility in Spain and the resulting product, which is shipped in a syringe, has a 48-hour shelf life. The therapy is injected directly into the lower limb, which means it is administered to the vessels directly where it is needed. The product is currently in Phase III trials and Rexgenero treated its first patient on this trial in February 2018.
As with all cell therapies, one of the major hurdles to wide-spread commercial uptake is not always the efficacy but the price tag.
“Currently, we’re aiming to reduce the cost per patient of REX-001 by more than 50 percent,” says Dr. Dupere. “To do this, we have been awarded an Innovate UK grant of £1.4 million to lead an industrial research project in a consortium with the Cell and Gene Therapy Catapult, TrakCel, and Thermo Fisher Scientific to design a cost-effective manufacturing strategy. If we can reduce the price enough, REX-001 has the potential to improve the quality of life for thousands of people every year.”






