Danqing Zhu Ph.D. Stanford University
Xinming Tong Ph.D. Research Associate Stanford University
Pavin Trinh Stanford University
Fan Yang Ph.D. Professor Stanford University
Stanford Engineers Successfully Encapsulate Cartilage-Forming Chondrocytes and Mesenchymal Stem Cells in 3D Hydrogels
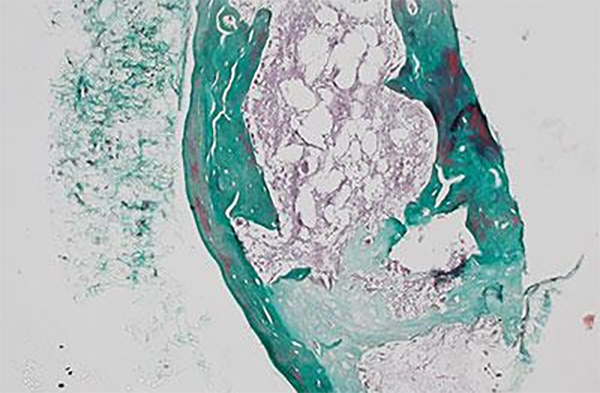
Despite the important role of zonal organization in cartilage structure and function, most tissueengineering strategies developed to date have only allowed the regeneration of cartilage with homogeneous biochemical and mechanical cues.1-5 Cartilage injury is extremely common, yet cartilage has limited self-healing capacity due to its low cellularity and avascular nature.1 As articular cartilage transitions from the superficial zone to the deep zone, the extracellular matrix (ECM) of the cartilage is characterized by increased stiffness, higher amounts of ECM constituents such as glycoaminoglycans, and increased presence of a hypertrophic cartilage marker such as type X collagen.6 Various strategies have been developed to generate biomaterials with gradient cues as cell niches,7 such as electrospinning,3 modular hydrogel system8 , laser-scanning lithography,4 convection-based diffusion,9 microfluidics,10 and fusing prefabricatd microspheres.11 However, most of these strategies often require lengthy fabrication processes or the use of reagents that are not cell-friendly, which do not support the fabrication of tissue-scale gradient biomaterials that allow direct cell encapsulation in a homogenous manner. As such, how gradient niche cues influence cell-mediated tissue formation in 3D remains largely unknown. In addition, although recent research has highlighted the role of mechanical signals, such as matrix stiffness, in modulating various cell fates including cell proliferation and differentiation,12-14 how activating or blocking mechano-sensing influences cell fate and ECM formation in 3D tissue remains to be examined.
To design zonal organization into engineered cartilage, one strategy is to spatially pattern chondrocytes from different zones with zonal-specific phenotype into stratified layers.15, 16 Using sequential photopolymerization, multi-layered hydrogels can be fabricated with chondrocyte subpopulations isolated from different zones and encapsulated in different layers to better mimic in vivo zonal organizations.2, 17 Another strategy is to spatially pattern matrix cues using multi-layered hydrogels with varying biochemical and mechanical properties using a single source of cells.18 Both biochemical and physical cues have been shown to play important roles in directing cell fates and tissue formation;19-21 both mesenchymal stem cells (MSCs) and chondrocytes encapsulated in multi-layered scaffolds with spatially varying matrix compositions and mechanical cues demonstrated zonal-specific differentiation.18, 22 While the above strategies have improved the zonal organization of engineered cartilage over that achieved with homogeneous hydrogels, the resulting tissues displayed distinctive layers, while native cartilage transition in a continuous gradient manner.23 Further, the interfaces between layers are often weak and susceptible to delamination under mechanical stress due to discontinuities in hydrogel stiffness at the interfaces.24
In contrast to multi-layered hydrogels, biomimetic gradient hydrogels present continuous changes in biochemical and/or mechanical cues, yielding more accurate mimicry of tissue interfaces and zonal organization in vivo. To induce zonal-specific differentiation of MSCs in 3D in order to better mimic the bone-cartilage interface, hydrogels containing soluble factor gradients were fabricated by encapsulating microspheres with recombinant human bone morphogenic protein 2 (rhBMP-2) and insulin-like growth factor (rhIGF-I) in a gradient manner.25 Additional work has also been done to fabricate hydrogels with different matrix stiffness with developmental signalling approaches to induce zonal-dependent MSC differentiation. 26 Attempts have also been made to fabricate hydrogels containing insoluble matrix gradients (for example, stiffness) using polyacrylamide (PAAM) hydrogels27, 28 with biochemical cues.29 A microfluidic-based method has also been explored to create dual gradient polyethylene glycol (PEG) hydrogels containing both chemical and physical gradients,30 but such micro-scale platforms are not suitable for fabricating tissue-scale gradient hydrogels for repairing tissues with clinically relevant dimensions. While cells reside in a 3D niche in vivo, previous reports were largely limited to 2D studies due to the challenge of encapsulating cells in tissue-scale 3D gradient hydrogels in a rapid and cell-friendly manner. As such, there remains a critical need to develop a biomaterials platform that enables the investigation of cell behavior in tissue-scale gradient hydrogels in 3D. The goal of the present study is to develop a cell-friendly method for fabricating tissue-scale gradient hydrogels as a 3D cell niche to guide regeneration of cartilage with zonal organization. We hypothesize that hydrogels with stiffness gradient would induce zonal-specific response of encapsulated cells in 3D, and the newly deposited tissues in gradient hydrogels mimics the zonal organization of native articular cartilage. To test this hypothesis, neonatal bovine chondrocytes or human mesenchymal stem cells were encapsulated in 3D gradient hydrogels for 7 or 21 days. Outcomes of newly formed tissues in different zones within gradient hydrogels were evaluated using mechanical testing, quantitative gene expressions, biochemical assays, and histology.
* Abstract
Zonal organization plays an important role in cartilage structure and function, whereas most tissue-engineering strategies developed to date have only allowed the regeneration of cartilage with homogeneous biochemical and mechanical cues. To better restore tissue structure and function, there is a strong need to engineer materials with biomimetic gradient niche cues that recapitulate native tissue organization. To address this critical unmet need, here we report a method for rapid formation of tissuescale gradient hydrogels as a 3D cell niche with tunable biochemical and physical properties. When encapsulated in stiffness gradient hydrogels, both chondrocytes and mesenchymal stem cells demonstrated zonal-specific response and extracellular deposition that mimics zonal organization of articular cartilage. Blocking cell mechanosensing using blebbistatin abolished the zonal response of chondrocytes in 3D hydrogels with a stiffness gradient. Such tissue scale gradient hydrogels can provide a 3D artificial cell niche to enable tissue engineering of various tissue types with zonal organizations or tissue interfaces.
Click to read the rest of this article and its references visit the original article.
Tissue Engineering, published by Mary Ann Liebert, Inc., a peer-reviewed journal, is the preeminent, biomedical journal advancing the field with cutting-edge research and applications on all aspects of tissue growth and regeneration. The above article was first published in the July 2017 issue of Tissue Engineering, Part A with the title "Mimicking Cartilage Tissue Zonal Organization by Engineering Tissue-scale Gradient Hydrogels as 3D Cell Niche". The views expressed here are those of the authors and are not necessarily those of Tissue Engineering, Mary Ann Liebert, Inc., publishers, or their affiliates. No endorsement of any entity or technology is implied.