AskBio founder R. Jude Samulski talks about the evolution and future of AAV gene therapy following his company’s blockbuster acquisition by Bayer in 2020.

It is rare that a breakthrough study performed as a graduate student sets the course for the rest of a scientist’s distinguished career. But Richard Jude Samulski’s work in the early 1980s on an emerging viral vector—adeno-associated virus (AAV)—played a profound role in shaping the evolution of the gene therapy field. Samulski and colleagues stuck to their beliefs in the clinical potential of AAV, riding out some difficult times for the field to emerge emboldened.
Until 2016, Samulski was the head of the Gene Therapy Center at the University of North Carolina School of Medicine. In October 2020, the German big pharma Bayer agreed to acquire Samulski’s company AskBio for an impressive $4 billion.
Kevin Davies recently interviewed Samulski to learn about his early research career highlights, the launch and evolution of AskBio, and his hopes for the future of AAV therapy. (This interview has been lightly edited for clarity and length.)
Kevin Davies: Jude, how did you first get interested in gene therapy?
Jude Samulski: You’re really going back in time! In 1978, I was a graduate student at University of Florida. I was the first student for a new professor named Nicholas Muzyczka. If you could spell his name, you can join his lab..! About a year or two into the project, it became clear that SV40 was very unstable due to recombination. So we started looking for a new virus as a gene delivery system.
That’s when we came across adeno-associated virus (AAV) as a unique virus. One, it infected humans. Two, it was non-pathogenic. And three, it looked like it had a unique life cycle: in one phase, it would go into the cell and be latent, which would be ideal for delivering genes. In the other phase, it required a helper virus to propagate itself. The key at that point was to see if we could segregate the production component from the latent component and manipulate it—produce virus in one setting and then have it deliver genes in another setting.
My job was to clone AAV into a bacterial plasmid and test whether the recombinant plasmid, when introduced into human cells, would mimic the latent phase, where it would be persistent until adenovirus co-infection. Then, when adenovirus came into the cell, it would activate AAV to go into the lytic phase and make copies of itself. We were able to do that in 1978—we published that in PNAS in 1982…1
I went on to do a postdoc at Princeton in Tom Shenk’s lab. We generated one of the vector substrates commonly used in every lab around the world for cloning genes into AAV. Our production system became very popular… We stripped it down—96% of the viral genome was removed. That made the packaging capacity of AAV about 5 kb that everybody uses today.
Davies: How excited were you about the clinical potential of AAV at this time?
Samulski: You have to appreciate, we were thinking about putting genes into cells, not people. The target was: can you get something back into the cell and validate it works? It was cells first and then animals next…
We realize[d] that by physical techniques, we could get the virus exposed to cells that it never saw in a normal infection. This opened up a plethora of target tissues simply based on AAV receptors—put it in the bloodstream it’ll go into the liver; put it into the hippocampus, it will go into neurons; AAV into the heart will go into cardiomyocytes. Everybody started studying diseases that were either tropic for ocular, liver, muscle, neuronal, and so forth. It really exploded.
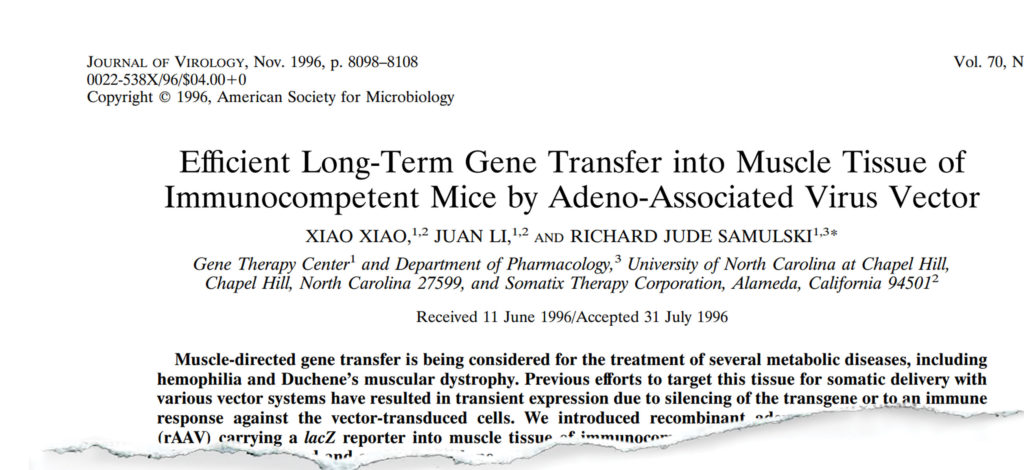
The paper that really launched a thousand labs to jump from retroviruses and adenovirus to AAV was the one that Xiao Xiao and we published in 1996.2 Realize this: everyone who put a gene into an animal’s muscle—whether it was adenovirus, retrovirus, lentivirus, plasmid, naked DNA or liposomes—they all had an immune response to the gene product and it was cleared from the tissue within two weeks to a month.
Xiao’s paper was the first example where we put in a gene and sacrificed the animal at two weeks, four weeks, etc.—each time he did it, he showed me the results using beta galactosidase… He went out over a year-and-a-half until they died of old age and said, “Jude, the gene is still on.” At that point, we knew this vector was special. This was that eureka moment… The promise of gene therapy was officially launched.
Davies: Where was that paper published?
Samulski: In the Journal of Virology in 1996.2 We could not get that paper published for two years! People wouldn’t believe it and each time we presented it, they kept saying there’s something wrong, you are staining the tissue wrong, etc. We went to a number of journals until we finally got some virologists that looked at it from a virology perspective. “Oh, it’s set up latency. That’s what one would expect.”
Davies: You founded AskBio back in 2001. How did you manage to launch a gene therapy company when the field was in such turmoil back then?
Samulski: Good question. Not only that, the day we launched it was 9/11. Talk about all the cards stacked against you! We did struggle. It was such a bad time with respect to gene therapy. People couldn’t segregate the difference between adenovirus and AAV after the death of Jesse Gelsinger. We immediately got thrown into that group of the “bad virus.”
We actually came up with a different name—we went around saying, “We don’t do gene therapy. We’re working on biological nanoparticles.” Nanoparticles were big back then; anything that was 20 nanometers or smaller was a nanoparticle. AAV was 20 nanometers and we published this in 2008.3
We said it delivers genes and they were all excited about it. It was a phase for a while until people became educated and realized that viruses were still the golden chariot as far as getting things to work with respect to efficient gene delivery. Most of our funding came from parents, foundations and SBIRs. Thank goodness for President Obama, because when the crash of 2009 happened, they were putting out money for small businesses and we were able to get some to keep our efforts going.
Davies: Your company is named AskBio for short, but tell us about the full name.
Samulski: It refers to Asklepios—the god of healing incurable diseases. In fact, the two snakes you see wrapped around the staff for the medical symbol [Caduceus]? That’s because one day, someone cut off the head of a snake and Asklepios supposedly put it back together and healed it…
In classic Greek mythology, Zeus was told by the god of death that [Asklepios] is taking his business away and he wants this guy taken out. Zeus decided, you can’t kill him. He’s doing something remarkable. Why don’t we make him into a god? So they turned him into the god of incurable diseases. We felt that was appropriate because we were working on incurable diseases. But we couldn’t say it or spell it, so we shortened it!
Davies: At some point you created subsidiaries—Bamboo, Chatham and NanoCor. I presume this was an important way, not only to specialize in certain therapeutic areas but also, once acquired, a great source of revenue?
Samulski: It was done for two reasons. I was part of the first wave of the gene therapy companies, but more as a consultant. I was part of a famous company that many people don’t remember called Somatix. Somatix had Inder Verma from the Salk Institute, Richard Mulligan from Harvard, Tom Shenk from Princeton, and they invited me to be part of that. They had retrovirus and adenovirus and naked DNA…
We failed miserably because we were ahead of our time. We were trying to develop these delivery systems and nobody was willing to fund because they were waiting for one delivery system to actually prove itself. When I saw that the whole company went towards cancer and then it was using adenovirus, and none of the technology we developed was ever going to be utilized, I backed off and said, I will never let that happen again.
The template we used was that AskBio would be the mothership. Rather than have a company take a clinical trial forward and, if it failed, the whole platform failed, we dropped it down into special purpose entities (SPEs) that went forward as Chatham Therapeutics or Bamboo or NanoCor. That way that structure could partner with someone and if it succeeded, it would go forward. If it failed, it didn’t kill the platform.
Davies: Did you consider taking AskBio public?
Samulski: We were getting ready to go public, but another variable that was hard to weigh in was being in the middle of a pandemic with ten million people out of work. How long is this going to last? What’s the market going to do? There’s a presidential election. We might go public and be dead in two months because all the money dries up.
When a number of large pharmas started knocking on the door saying, “we’re interested,” we saw that as an opportunity for our funding entity. If they leave us alone, we get everything we wanted as if we went public. It turned out to be more conducive for us being independent and not having to flow with the markets to see what would happen.
Davies: Bayer’s deal was reportedly for $4 billion, but you’re being left as a fairly autonomous unit within the pharma?
Samulski: We only did it if we were autonomous. They were very insightful saying, “We want to leave you alone because you’re innovators and we need you to keep doing that. We don’t want to burden you with our bureaucracy and processes.” That was a tremendous opportunity to finally get enough gas in the engine that you could drive the whole track without having to stop.
Davies: What do you have in the pipeline and what does this infusion of funds allow you to do that perhaps you couldn’t execute before?
Samulski: Almost everything we did before was [funded by] a foundation or family or a grant. That meant you did one project at a time and try to get it in the clinic. Now we have Pompe disease—we’ve treated six patients. The heart trial has treated three patients. We have a Parkinson’s trial by Krys Bankiewicz that’s treated 16 patients, and a trial lead by him for amino acid decarboxylase [deficiency] that has treated around 23 children.
Let me make this clear: We wanted to make sure that our technology, which we’re hoping will address many patients’ needs, didn’t turn into a technology for only those that have money, or only for diseases that are prevalent enough to be marketable. So we started a foundation called the Columbus Children Foundation. We treat diseases with prevalence of under 100 patients total in the foundation with the same technology that we’re developing for the larger mass markets.
Right now, amino acid decarboxylase deficiency has about 90 patients in the world. This is a form of juvenile Parkinson’s—these kids are born frozen. Krys Bankiewicz is the primary person doing all these trials. We’ve now treated 26 children in Warsaw, Poland, and in the U.S. We hope that this is the first disease that gets eradicated by gene therapy. The Foundation’s goal is to systematically knock these diseases with smaller prevalence out one by one. We see the Foundation as a mechanism to treat ultra-rare diseases that, while we’re developing through AskBio drugs for larger markets like Pompe or hemophilia. Both use the same technology, just under different structures. If you try to develop gene therapy drugs for ultra-rare diseases with the goal of making money, you’re never going to be successful. So we’re funding the development of ultra-rare diseases by a different mechanism. Bayer was tremendously behind it and said keep going.
Davies: AAV has an excellent safety profile, but last summer came news about adverse events in clinical trials. Is there more work to be done to ensure that AAV is as safe as it can possibly be in a clinical setting?
Samulski: You need to be aware of the following data that led to where we are today. The Avigen trial done by Kathy High showed that in a human, if you put in 1012 AAV particles per kilo for hemophilia indication, they saw liver toxicity. Everybody backed up saying, “this concern was not obvious in the animal models at any level. What’s going on here?”
While people were trying to figure out that immunology and suppressing it with steroids, we developed the self-complementary vectors which would allow you to drop down in dose and achieve the same therapeutic level with a much lower particle number.
It was Brian Kasper and Jerry Mendell at Ohio State that broke the sound barrier of the gene therapy field when they went into SMA (spinal muscular atrophy) children with 1014 AAV particles per kilo. We were scared to death that it would be an all-out toxicity problem. When they were able to manage that and still be able to cure these children, everybody decided the new ceiling went from 1012 to 1014.
We have two logs of toxicity that we’re playing around with. We know if we stay below that we’re safe. Anything above that, you’re running a risk.
The full version of this interview was originally published in Human Gene Therapy (January 2021).
References
1. Samulski RJ, Berns KI, Tan M & Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. PNAS 1982;79:2077-2081.
2. Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virology 1996;70:8098-8108.
3. Li W, Asokan A, Wu Z et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Molecular Therapy 2008;16: 2052-2060. doi: 10.1038/mt.2008.100.Epub