May 15, 2014 (Vol. 34, No. 10)
Pete Gagnon CSO BIA Separations
Novel Operating Mode for Carrying Out Anion Exchange Chromatography
Anion exchange chromatography (AEC) is one of the pillars of downstream processing, especially for IgG purification. Its ability to simultaneously reduce DNA, virus, and host protein contamination has made it an essential polishing tool, and it is the only fractionation method used in more processes than affinity chromatography.
Thus, AEC is a natural center for innovation. Physical advances with membranes and monoliths have created new capabilities, but novel techniques continue to evolve for columns packed with porous particles as well.
The classical operating modes for AEC are bind-elute (BE) and flow-through (FT). With BE, the product of interest binds, while weaker-binding contaminants wash through to waste. The product is then eluted while stronger-binding contaminants remain on the column. BE has particular utility for purification of mouse IgG monoclonals because they typically have sufficient negative charge to bind with fair capacity under mildly alkaline conditions at low conductivity.
Human IgG1 monoclonals tend to be sufficiently alkaline that it is difficult to obtain high capacity in BE mode, often requiring excessively high pH and low conductivity, and still achieving low capacity. However, their higher isoelectric point also means that a larger proportion of the overall contaminant load is acidic compared to the antibody, which favors effective fractionation in FT mode.
FT mode is also simpler than BE because it does not require development of elution conditions, and thereby supports the additional benefit of reducing the total number of process buffers.
High-performance tangential flow filtration (HPTFF) represents a more recent operating mode where electrostatic repulsion between an alkaline antibody and the positive charges on a membrane prevents antibodies from passing through pores that are physically large enough to permit their passage.
Smaller electroneutral contaminants are eliminated through the pores. Acidic contaminants are retained, but they also gradually neutralize the positive charge on the membrane and limit capacity. Up to that point however, HPTFF can also permit the antibody to be concentrated.
Void Exclusion Mode
The fact that charge repulsion can be used to control selectivity on a membrane suggests that it must also contribute to protein behavior on columns packed with porous particle anion exchangers. Recent data confirm this expectation. Alkaline proteins like IgG antibodies are restricted to the void volume.
The void volume represents the shortest path through the column, so IgG elutes first, nearly pure. This is the basis for naming the technique Void Exclusion (VE) mode. Weakly alkaline and neutral contaminants, and small molecules, including buffers, salts, and media components, diffuse through the pore volume of the column and exit later. Acidic contaminants bind, and are removed in a regeneration step (Figure 1).
VE supports 99–99.9% host protein reduction in a single step from whatever sample is applied to it. It simultaneously supports a 4–5 log10 reduction of DNA, 3.0 log reduction of murine leukemia virus (MuLV), and 3.5 log reduction of minute virus of mice (MVM). It also supports 98–99% IgG recovery with less than 10% dilution from the applied sample volume, due to the fact that the IgG never comes into physical contact with the surface and does not diffuse into the pores.
In dramatic contrast to all other modes of AEC, VE tolerates any sample pH and conductivity. This derives from its ability to perform buffer exchange in parallel with anion exchange, and eliminates any need to dilute the sample or perform an intermediate buffer exchange step in preparation for the next process step.
VE particularly enables the option of using AEC as a seamless bridge between two other methods, such as between protein A affinity and cation exchange chromatography.
Also distinct from other modes, capacity of VE is not mass limited. IgG can be applied at any concentration. Instead, capacity per cycle is volume limited. As with buffer exchange by size exclusion chromatography (SEC), sample volume is limited to the interparticle volume of a column; the void.
As a practical matter, this means that industrial applications will require multiple cycles to process an entire product lot, but this fits well with the growing trend of continuous processing methods. Also as with buffer exchange on SEC media, column packing matters. Columns should be gravity packed. Axial compression of the bed diminishes the void volume, and capacity with it.
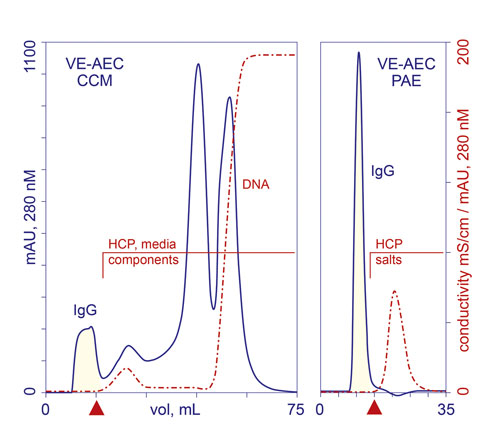
Figure 1. VE-AEC profiles from fractionation of cell culture harvest clarified by centrifugation and microfiltration (CCM), and IgG eluted from protein A (PAE). HCP: host cell protein. Red triangles mark the end of IgG collection.
Method Development
Choice of AEC media for practicing VE is limited. Membranes and monoliths do not work because they lack a void volume, but even among porous particle AEC media, few suitable candidates have been identified. Comprehensive characterization of UNOsphere™ Q (Bio-Rad Laboratories) has revealed charge-rich low-density-polymer zones supported by a high-density-polymer particle skeleton.
Electropositive proteins are repelled while the physical spacing of the polymer strands in the low-density polymer zone prevents entry of electroneutral proteins, including IgGs at pH values very close to their isoelectric point. Acidic contaminants are drawn into the charge network whatever their size and remain bound there until eluted.
Selectivity is otherwise determined by the properties of the antibody and the equilibration buffer. Highest purity is obtained at the highest pH and lowest conductivity where the antibody does not bind. 20–50 mM Tris, pH 8.0 is a good starting point. Higher pH can be explored if the antibody does not bind. Lower pH or addition of salt can be explored if it does.
VE requires an elution step to restore contaminant-binding capacity for the next cycle, but it can be embedded in the sample composition. Simply add excess salt to the sample so that when the sample salts migrate down the column, they elute contaminants that may have bound to the media. This eliminates the need for a distinct elution step and reduces the number of process buffers, as well as the overall process volume.
This leaves only the requirement for a regeneration-sanitization buffer such as 1 M NaOH. In most cases, NaOH need be applied only once per product lot, after the last process cycle.
The high degree of single-step purification offered by VE invites the idea that it could be used as a replacement for protein A, with the obvious benefits that the chromatography media is dramatically less costly, and supports rigorous sanitization with 1 M NaOH. This option is limited by the fact that excess free light chain and light chain dimers produced by some cell cultures tend to co-purify with IgG (Figure 2).
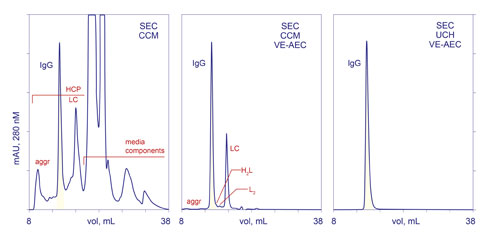
Figure 2. Purification process comparison. SEC-CCM: analytical SEC of harvest clarified by centrifugation and microfiltration. SEC-CCM-VE-AEC: analytical SEC of harvest clarified by centrifugation and microfiltration, then purified by VE-AEC. SEC-UCH-VE-AEC: analytical SEC after ultra-clarification of harvest, then VE-AEC. HCP: host cell protein. L, LC: light chain. H: heavy chain. aggr: aggregates.
One study conducted with biosimilar Herceptin® demonstrated that these contaminants could be removed by a polishing step with cation exchange, hydroxyapatite, or Capto™ adhere (GE Healthcare).
Subsequent work has demonstrated that when harvest is clarified by a method that removes most of the excess light chain, VE reduces host protein contamination to less than 50 ppm. A final polishing step with Capto adhere step reduces it to less than 1 ppm, and concurrently reduces aggregates to less than 0.05%. The overall process economics of VE as a first column step make it 2–3 times less costly than protein A (Table).
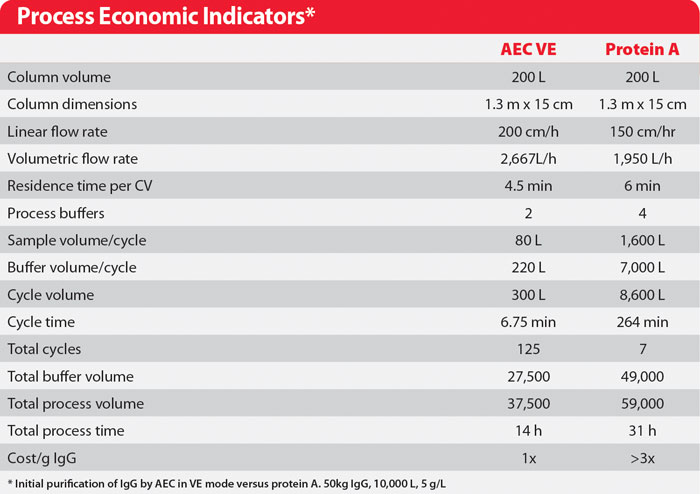
Table. Process Economic Indicators*
Pete Gagnon ([email protected]) is group manager for downstream processing at the Bioprocessing Technology Institute in Singapore.







