April 1, 2018 (Vol. 38, No. 7)
At present, most of the commercially available cancer immunotherapies employ monoclonal antibodies or cancer vaccines, but a different kind of cancer immunotherapy, adoptive cell transfer (ACT), is progressing rapidly. ACT usually involves the harvesting of patients’ own immune cells, expanding these cells (or engineered versions of them), and then reintroducing them to patients.
ACT-based treatments pose serious developmental challenges,img not the least of which is the need to evaluate the cancer-killing abilities of the immune cells. In addition to being robust and simple, the ideal in vitro assay closely mimics activity in vivo. The ideal in vitro assay must also predict long term behavior in animal models, and ultimately, in human patients. To facilitate the study of immune cell–mediated killing, ACEA Biosciences – now a part of Agilent Technologies – has developed the xCELLigence® Real-Time Cell Analysis (RTCA) platform.
The RTCA platform is described below, the main text of which is devoted to showing how the RCTA platform is helping advance real-world projects. In this article, several key opinion leaders tell GEN how they are using the RTCA platform.
More detailed case studies and deeper discussions will take place during the “Cancer & Immunotherapy Symposium 2019.” Hosted by ACEA Biosciences, this event will be held March 30 in Atlanta, GA, in advance of the annual meeting of the American Association of Cancer Researchers (AACR). For more information visit www.aceabio.com/cancer2019.

GEN: Why is there such a great disparity in the potency of current cancer immunotherapies between patients or between patient subpopulations?
Dr. Anderson: Two factors, genetics and the tumor microenvironment, are major focuses of investigation. In addition, adaptive resistance is not very well understood yet, but it’s going to be a problem for many types of immunotherapy. Cancers have adapted to evade treatment strategies in the past, and we’re now seeing that this is true for immunotherapy as well. We’re trying to predict how tumors might evade our therapies so we can develop strategies to head that off.
Dr. Bamdad: I believe the underlying problem is how the cells are being handled in vitro before they’re given back to the patients. It’s looking more like the longer they’re cultured in vitro, the worse it is for the patient, because as these cells mature, the distribution of the different subpopulations of T cells changes. This has a negative impact on persistence (that is, how long they will be effective in the patient) and also on what is called the cytokine storm, or neurotoxicity. Our understanding of this topic is increasing exponentially.
Dr. MacLeod: In CAR T cell– and other T cell–mediated therapies, one of the big reasons for variability is the use of a patient’s own cells. Instead of taking an autologous approach, at Precision Biosciences, we are engineering healthy donor cells to transfer into unrelated patients, and this allogeneic approach allows us to make something more defined, more consistent. This is evident when we use assays to evaluate the function of these products such as the cytotoxicity assays developed by ACEA Biosciences for the xCELLigence instrument.
Dr. Golubovskaya: Patients differ in their immune profiles, and tumors differ in the biomarkers they present. We cannot predict these differences, and we rely on analyzing these biomarkers in each patient to help us predict their response to immunotherapy.
GEN: What are the main challenges in assessing and monitoring the potency and efficiency of cytotoxic immunotherapies targeting cancer?
Dr. Anderson: The challenges differ for each tumor type. Most experiments that work with human samples have to be done in a Petri dish, which does not replicate the complexities of the tumor microenvironment. In addition, we have had problems looking at ovarian cancer cells using some of the “gold standard” assays, such as chromium release and flow cytometry killing assays. The cancer cells we use often do not take up or retain the chromium label well, resulting in high background readings.
With flow-based assays, which also require labeling, if you put in too many cell types, which are often necessary as controls, overcrowding can occur and result in nonspecific tumor cell death. In these scenarios, background cell death makes it difficult to evaluate true T cell–mediated killing.
Dr. Overstreet: One of the biggest challenges is to improve our ability to model and predict what will work in humans. Many of these pathways are understood in the mouse, and while some of them have provided good translation to humans, others have not. We have been able to build in vitro systems on the xCELLigence platform in which we can study the cognate interactions between human T cells and human tumor cells in a way that might be more reflective of what you would see in a patient’s tumor.
Dr. Bamdad: Timing, accuracy, and flexibility of your instrumentation are paramount. You can use fluorescence-activated cell sorting (FACS) to look at CAR T-cell killing, but it gives a very indirect measure, and it lets you look only at a snapshot in time. You can’t see how your CAR T cells or cancer cells are evolving over time.
You need a technology that allows you to look at the co-culture of your cancer cells and CAR T cells over time to be able to identify the correct distribution of naïve or central memory to effector memory T cells. You need to be able to determine how long it takes these cells to mature so you get to see when cell killing occurs, on the opposite end of the spectrum, when CAR T cells become exhausted and the cancer cell population starts to grow again. Being able to see what happens in real time is critical to your CAR design and how you culture your CAR T cells in vitro to get the proper subtype distribution.
Dr. MacLeod: One of the main challenges is being able to translate the in vitro results to how the therapy will actually perform in vivo, either in animal models or in patients. The traditional assays for measuring cytotoxicity are usually short, sometimes 3 or 4 hours, and to see a response, you use very high effector-to-target cell ratios. This will show activity, but it doesn’t reflect the entire mechanism of action.
These are living products—they don’t just kill cells, they also proliferate, and that’s an important part of the mechanism. When you are able to look at killing of target cells over longer periods of time at lower effector-to-target ratios, that allows the effector cells to proliferate and kill multiple targets—what is called serial killing—and that type of longer assessment is a much more stringent way to evaluate T-cell activity.
GEN: Why is it important to measure target cell killing directly rather than by quantifying related factors such as levels of secreted compounds or the activation of effector cells?
Dr. Anderson: Both are important, but it’s especially important to measure target cell death because every tumor is a little different. Some tumor cells may respond to one killing mechanism and not to another, and because they are also so readily able to adapt to evade T cell–mediated killing, we need to be able to see that the T cells can actually kill the targets.
It is important to note that the xCELLigence assay isn’t technically a direct readout of cell killing, it’s a measure of impedance, which reflects the ability of the target cells to adhere to the bottom of the plate. Since dead cells do not impede the signal, this is an indirect readout of cell killing. For the purposes of our experiments, it has yielded the most reproducible results and allowed us to study physiological effector-to-target ratios that we couldn’t evaluate using the other assays.
Dr. Overstreet: If what you ultimately want to see is how a T cell interacts with a tumor cell and kills it, then that’s what you should be measuring. T cells have an array of overlapping/semiredundant ways of killing a target cell, and by choosing to measure a single analyte, you could be missing the critical limiting factor in the particular interaction. But if you look at how a tumor cell is dying in real time, then you can see that dynamic interaction that is the net result of all the effector functions, and you can observe how your therapeutic can alter that interaction.
GEN: In the design and optimization of a CAR T cell– and other T cell–mediated therapies, what are the most crucial characteristics of an assay method used to analyze immune cell–mediated killing kinetics?
Dr. Anderson: Sensitivity, accuracy, and reproducibility are critical. One advantage of a high-throughput technology is the ability to include many controls, which are necessary to ensure that what we are seeing is actually T cell–mediated killing. The xCELLigence instrument also allows us to monitor the health of the target cells, and the software corrects for nonspecific background killing.
Dr. Overstreet: One advantage of xCELLigence assays compared to traditional means of evaluating T-cell toxicity is the greater specificity. Given that we’re able to monitor in real time over 3–4 days with these assays, instead of capturing a single time point over 4–6 hours, we can lower our effector-to-target cell ratios down to 0.5–2:1—approaching a 1:1 interaction between T cells and tumor cells. You see slower, steadier killing, which allows for the back-and-forth communication between a tumor cell and a T cell that might be more reflective of what you see in a tumor. At those lower effector-to-target ratios, the specificity of the killing is also improved—dramatically increasing dynamic range.
Dr. Bamdad: The ability to simultaneously test a number of different conditions is important. Using FACS, which is a sequential method, it would have taken us 6–9 months to evaluate our 60 different CARs against various types of cancer cells under different conditions. Using our 6 × 96–plate xCELLigence platform, we were able to analyze 576 conditions in parallel, enabling us to complete this work in less than a month. We are also able to add reagents to the experiments while they’re in progress and assess the effects on cancer killing in real time.
Dr. Golubovskaya: Being label-free, real-time, dose-dependent, and time-dependent are the most important characteristics for immune cell–mediated killing assays. The xCELLigence technology is very good because it is measuring the cytotoxicity of the T cells and the death of the target cancer cells in real time.
GEN: What are the specific advantages of the xCELLigence instrument and the benefits of a label-free assay method? How would you describe its role in early-stage research and commercial product development?
Dr. Overstreet: I’ve had requests from scientists throughout our organization interested in using the instrument because it is so data rich with its real-time analysis. The label-free aspect of the xCELLigence platform streamlines your workflow. Also, the platform’s nondestructive analysis allows you to perform any kind of orthogonal readouts to assess the antitumor responsiveness of your T cells, such as flow cytometry or cytokine analysis.
Combining this instrument with some of the scientific infrastructure and human immunology model development that we’ve done here, a lot of people are really excited about our approach and our ability to test molecules in a relevant human cell–based system. We hope this will ultimately improve our preclinical modeling and strengthen our rationale for taking molecules into the clinic.
Dr. Bamdad: We are working with the Fred Hutchison Cancer Center, and we expect to start human trials later this year. As you near clinical trials, you have to bridge the gap from working on the benchtop and in animals to treating patients. Every CAR T cell and tumor cell we put into animals we look at in parallel on the xCELLigence instrument. So far, the results have perfectly mirrored the results we’re getting in animals. That gives us more confidence as we’re modifying what we do to move toward human testing.
Dr. Golubovskaya: The advantages and benefits of using a label-free assay are as follows: fewer steps, fewer variables, and a more direct assay. We have the xCELLigence system with 6 × 96–well plates and are measuring the cytotoxicity of CAR T cells against cancer cells in real time. We plate different types of cancer cells on day 1, and the next day we add our effector CAR T cells at different effector-to-target ratios, allowing us to analyze cell killing at different time points and at different doses. The system’s software allows you to quantify the assay at any time point, so you can see the kinetics of killing. It’s a very visual, convenient assay.
Dr. MacLeod: The biggest benefit is simplicity, which makes the assay easier to run, a little more consistent, and easier to teach to new users. We’ve mainly been using the instrument at a very early stage, when we’re just testing out a large number of different CAR T constructs. The sensitivity of the assay allows us to use very few cells, and because we can detect killing at low effector-to-target cell ratios, we are able to do a lot more screening.
Game-Changing Platform Speeds Discovery and Development
Critical to the design and development of effective immunotherapies such as chimeric antigen receptor (CAR) T cells, checkpoint inhibitors, and oncolytic viruses, is the ability to monitor the potency of treatments against target tumor cells in vitro. In addition to being robust and simple, the ideal in vitro assay should also be highly predictive of how the therapy will behave in animal models and, ultimately, in human patients.
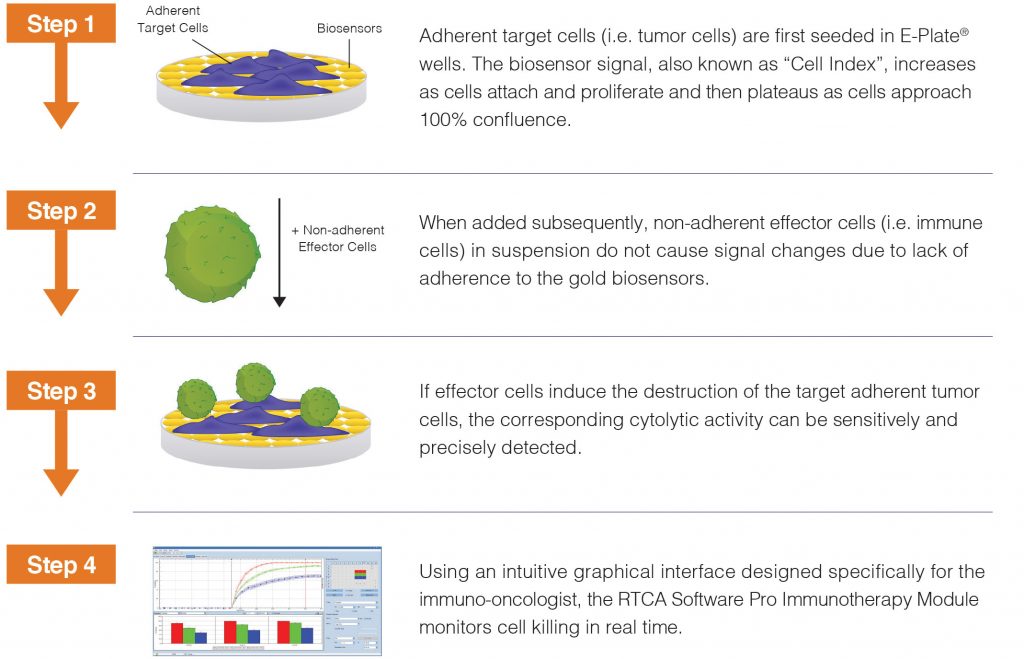
To more closely mimic activity in vivo, ACEA Biosciences – now a part of Agilent Technologies – has developed the xCELLigence® Real-Time Cell Analysis (RTCA) platform to quantitatively monitor cancer cell killing over extended time periods. This label-free and noninvasive technology captures the response of adherent tumor cells to different treatments through proprietary biosensors embedded into custom microtiter plates.
The workflow is remarkably simple: plate and incubate target tumor cells, add effector cells to be tested, then let the xCELLigence platform monitor tumor cell killing without further human intervention. Although liquid tumors (that is, blood cancers) are not naturally adherent, they can also be monitored by the xCELLigence platform through ACEA Biosciences’ antibody-mediated tethering kits for use with these custom plates.
The straightforward workflow makes it easy to analyze multiple parameters simultaneously, such as different doses of checkpoint inhibitors or variations of CAR constructs. The high sensitivity of this assay also enables low, physiologically relevant effector-to-target ratios to be examined, and its continuous data monitoring yields a complete and nuanced view of cancer cell killing that is missed by endpoint data.
An example of how the xCELLigence system is being used to develop and optimize CAR-T cell immunotherapies was recently published by Carl June and colleagues from the Perelman School of Medicine, University of Pennsylvania. The researchers used the xCELLigence platform to measure the lysis of non-small cell lung tumor cells treated with CAR T cells at a 1:1 effector-to-target cell ratio over a 20-hour period (Guedan S, Posey AD, Shaw C, et al. JCI Insight 2018; 3[1]: 96976).
Download the Cancer Immunotherapy Handbook to discover new quantitative methods to monitor immune cell behavior in vitro.
Applications in the Cancer Immunotherapy Handbook include CAR-T cells, Checkpoint Inhibitors, Antibody-Dependent Cell-Mediated Cytolysis (ADCC), Bispecific T Cell Engagers (BiTEs), Bispecific Antibodies, Oncolytic Viruses, Liquid Tumor Killing, and more. To gain a more complete view of cancer cell killing, the xCELLigence® Real Time Cell Analysis (RTCA) assay captures dynamic cell behavior that is missed by endpoint methods.






