February 15, 2016 (Vol. 36, No. 4)
Engin Ayturk Ph.D. Senior R&D Manager Pall Life Sciences
Chris Forespring Senior Manager, Scale-Up and Process Engineering Team MedImmune
Aiming for Robust, Flexible Bioprocesses with Solutions that Deliver Consistent Product Quality
Smaller, modern multiproduct biopharmaceutical production facilities require greater flexibility and higher throughput at lower cost, so many manufacturers are responding with the implementation of continuous processing and single-use technologies. Continuous upstream perfusion processes can, however, create downstream bottlenecks in tank capacity for manufacturing plants designed for fed-batch systems or with limited footprints.
Concentration using tangential flow filtration (TFF) can help to some extent, but it involves recirculation of the product solution, which can be damaging to sensitive biotherapeutics. New, in-line single-pass TFF (SPTFF) disposable technology does not suffer from this limitation, and can be operated virtually anywhere within a given bioprocess.
Benefits of Continuous Processing
The transition from batch to continuous bioprocessing offers numerous advantages, including process time savings and lower capital costs due to smaller equipment, tanks, and tubing sizes. In addition, some hold-tanks between unit operations may be eliminated, which is particularly valuable for facilities with limited manufacturing floor space. Furthermore, reduced system holdup volumes improve product recoveries and contribute to the smaller footprints of continuous bioprocessing systems. As importantly, continuous manufacturing typically results in more consistent product quality; requires less energy, water, and raw materials; and produces less waste. Less human intervention is also generally required, which reduces the risk for human error and contamination.
The smaller scale of continuous processes also enables the use of disposable technologies, which in turn can increase the flexibility and throughput of manufacturing facilities. Specific benefits of single-use systems include reduced cross-contamination risks; shorter setup times; elimination of cleaning and cleaning validation steps and costs; and lower capital and consumable expenditures.
Continuous Processing with SPTFF
SPTFF technology from Pall eliminates the conventional TFF recirculation loop and uses a staged flow-path design, allowing concentrations of 2–30x to be achieved continuously in a single pass. The single-pump pass reduces minimum working volume concerns and is ideal for the processing of fragile biomolecules. Disposable SPTFF in-line concentration (ILC) modules use the same staged flow-path design combined with a built-in restrictor for greater control. They also achieve concentration factors (2–4x or higher) suitable for in-line volume reduction of typical monoclonal antibody (mAb) or other biopharmaceutical (antibody fragment, enzyme, etc.) solutions in a single module-pump pass.
Compared with conventional TFF, SPTFF not only eliminates the recirculation loop, it provides greater flow ratio control, a 5–10x lower feed rate, 2–3x smaller lines sizes, lower working volumes for easier product recovery, low shear exposure, and there are no mixing or foaming issues. Under optimized conditions, product recoveries are typically above 99%.
ILC via SPTFF can be readily coupled with most downstream bioprocess operations to help overcome facility constraints on the number and volume of product tanks. Common applications include but are not limited to post-harvest volume reduction, concentration prior to capture chromatography, in-process volume reduction and/or concentration, in-process dilution/de-salting, and final concentration (replacement of a second ultrafiltration (UF2) step).
Pall has demonstrated that in-line concentration using SPTFF is a reliable, scalable technology suitable for R&D; process development; and pilot and production scales that also enable continuous processing by coupling downstream operations. In this context, SPTFF technology is not only an important addition to the process development toolbox for platform process evaluation, but also a crucial enabler of integrated, streamlined, and continuous bioprocessing initiatives.
The advantages of adopting SPTFF at the process development, pilot, clinical or commercial manufacturing scales over conventional approaches can be summed up as follows. It:
- Provides flexible manufacturing through process integration/coupling and continuous upstream and downstream processing;
- Reduces processing volumes, system sizing and facility footprint; leads to novel facility fit solutions; and overcomes manufacturing bottlenecks;
- Enables disposable and/or single-use technology utilization, thereby increasing productivity, eliminating nonvalue-added processing steps and improving workflow;
- Achieves high product recoveries and increases yield through utilization of smaller, more compact systems;
- Enables high concentration factors and the processing of highly shear-sensitive products; and
- Provides major savings with respect to capital expenditures, materials, labor, and facility operating costs.
Simplifying Bioprocessing with SPTFF
ILC with disposable SPTFF technology was seen as a potential solution for overcoming facility constraints and increasing the flexibility and throughput of operations at a leading global biologics manufacturer. The performance of ILC modules was therefore evaluated in key downstream processing steps in order to determine its feasibility over a wide range of feed streams at varying concentrations and process temperatures, as well as to identify appropriate module sizing and process benefits at commercial scale.
Perfusion Recovery
Perfusion culture processes create downstream bottlenecks in tank capacity for manufacturing plants designed for fed-batch processes. ILC of clarified culture fluid (CCF) allows the perfused volume to be collected in an existing tank and batch-processed downstream without facility modifications. Processing at the reactor temperature (37°C) was found to increase the convective transport and overall throughput by 30% volumetric concentration factors (VCF = 3.2x) compared with the results obtained under ambient conditions (21°C). The VCF was then further increased by applying additional backpressure to the ILC module. Overall, it was determined that currently available ILC modules can process up to 2,000 L bioreactor volumes operated in continuous mode and provide a streamlined solution to facility-fit constraints (Figure 1).
Pre-Capture Chromatography
For mAb processes, protein A chromatography is commonly used as a capture step. Due to the high cost of this resin, it is desirable to reduce the packed bed volume (PBV) and perform multiple cycles, thus exchanging raw material cost for operational cost. However, the lower volumes and higher titers obtained following ILC via SPTFF lead to shorter load times, which allows for both reduced raw material and operational costs.
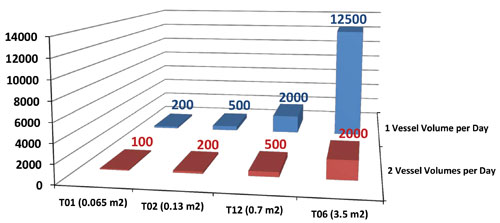
Figure 1. ILC Sizing chart for perfusion process. Back-pressure excursions at ambient temperature were performed while maintaining a constant feed pressure of 60 psig.
In one case, Pall’s customer determined that after ILC the increase in total processing time due to the use of a reduced column volume was shifted by as much as a factor of two. Overall, the PBV was decreased from 57 L (60 cm) to 31 L (45 cm), saving $416,000 in raw material costs. In addition, a 6x concentrated CCF loaded onto a protein A capture column exhibited no difference in eluate CHOP, DNA, percent yield, or mAb purity compared with the control.
At the present time, the sizes of available ILC modules limit chromatography operations to 20 cm diameter columns. However, when coupled, back-pressure to the ILC module is expected to increase the VCF, which will help achieve a higher throughput (Figure 2).
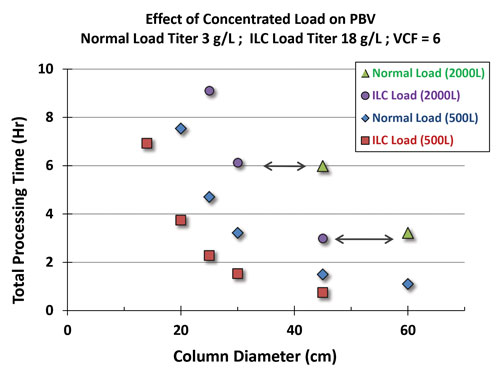
Figure 2. Effect of concentrated load on PBV
In-Process Volume Reduction
Purification of biopharmaceuticals consists of multiple chromatography steps combined with additional filtration and treatment steps to remove and inactivate viruses. Linkage of these steps occasionally requires adjustments or dilutions that can pose facility fit constraints. The use of ILC can decrease pool volumes to enable increased disposables usage and to provide options for increasing the operability range of manufacturing plants.
Studies by the global biologics producer revealed that the feed concentration impacts the VCF and throughput obtained at a given operating pressure. In addition, the reproducibility and responsiveness of SPTFF systems to changing operating conditions were confirmed. When used to concentrate a chromatography column effluent, it was found that less ILC area was needed, but that back-pressure from the ILC may impact column operation. When used prior to membrane chromatography, ILC decreased both the processing time and pool volume.
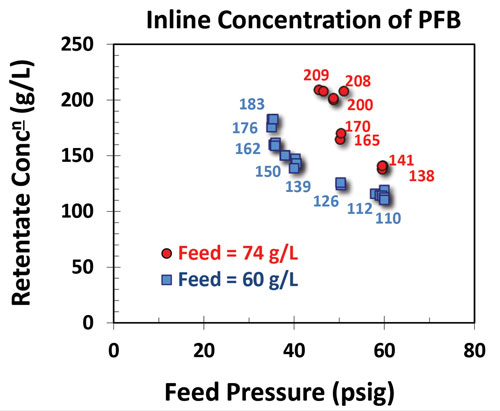
Figure 3. Inline concentration of PFB
Ultrafiltration/Diafiltration (UF/DF)
UF/DF system design is critical to facility fit for a multiproduct facility. However, because a wide range of process titers and drug substance (DS) concentrations are processed in such a facility, it is difficult to design a one-size-fits-all UF/DF system. As a result, some facilities employ two UF/DF systems (UF2) to achieve high DS concentrations (>150 g/L), which increases their costs and the process complexity. Pall’s customer found that replacing a UF2 system with an SPTFF ILC system reduced the required capital investment while providing lower holdup volumes and higher recoveries with reduced overconcentration targets and minimal pump passes. It was also determined that currently available ILC modules are suitable for processing 12,000 L reactor volumes up to 2.7 g/L titer in three hours (Figures 3 and 4).
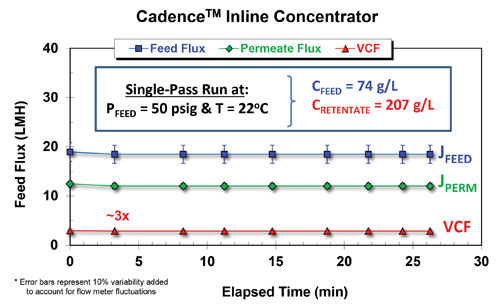
Figure 4. Performance exhibited by the CadenceTM Inline Concentrator
Conclusions
Studies revealed several important and positive findings regarding the use of SPTFF for ILC of biopharmaceutical solutions during commercial-scale downstream processing:
- 3–8x VCF were demonstrated for a wide range of feed streams and concentrations;
- No difference in product quality (mAb purity, enzyme activity) was observed under the various conditions employed;
- Currently available ILC modules fit perfusion process flows of 1–2 vessel volumes per day for reactor volumes from 100 L to 2,000 L;
- The use of SPTFF for ILC before capture chromatography has the potential to save significant cost while maintaining acceptable processing times;
- Concentration using SPTFF in line with chromatography operations is feasible, and may enable improved chromatographic selectivity through the use of shallow gradients; and
- SPTFF as a replacement for UF/DF can alleviate the working volume constraints of conventional systems, and thus potentially decrease overconcentration minimums, increase recoveries, and improve the operating ranges of existing systems.
These results confirm that SPTFF systems can enable biopharmaceutical manufacturers to achieve greater flexibility and higher throughput at lower cost, facilitating continuous bioprocessing and maximizing the operational use of facilities with smaller footprints.
Engin Ayturk, Ph.D. ([email protected]), is senior R&D manager, biopharm applications, integrated continuous bioprocessing at Pall Life Sciences. Chris Forespring is senior manager, scale-up and process engineering team in bioprocess engineering within the development organization at MedImmune.







