May 1, 2013 (Vol. 33, No. 9)
David A. Mann, Ph.D.
Shannon Einhorn
Kristin Block
Peter Fuhrken, Ph.D.
Christian Kannemeier, Ph.D.
Jen Luebke-Wheeler, Ph.D.
Functional Model Tissue for In Vitro Predictive Metabolism, Toxicity, and Disease Modeling
The liver is a complex organ composed primarily of hepatocytes, along with supporting vascular tissue and Kuppfer cells. It is responsible for normal metabolic functions and is also critical for the processing of xenobiotics taken in from the environment. Liver damage from viral assault and other types of toxin-mediated injury can lead to disease states resulting in reduced hepatic function and even death. The study of hepatic functions are thus of keen interest to areas of environmental toxicology, disease modeling, and drug development.
Existing in vitro models of liver function currently employ immortalized cell lines, such as HepG2, HepaRG, and Huh7, as well as primary tissue isolates from human and animal donors that can be studied as intact liver slices or though the dissociation and isolation of primary hepatocytes. Limitations of these cell and tissue models include reduced function in culture that may also change with passage number.
Primary tissues can be supply limited and also represent a variable source of functional material as there can be dramatic differences in phenotypic responses to stimuli based on the genetic background and environmental exposure history of the donor. For isolated primary cells, this heterogeneity can be mitigated by pooling donor tissues to generate a mixed sample of tissues for study, but this can still yield batch-to-batch variability in culture.
Commercial Hepatocytes
To overcome the limitations in supply, culture stability, and inconsistent function, recent efforts have produced and made available human hepatocytes differentiated and matured from induced pluripotent stem cells (iPSCs). iCell® Hepatocytes, currently available from Cellular Dynamics International (CDI), are human iPSC-derived hepatocytes generated from a single genetic background, enabling consistent performance, produced in limitless supply, and offering superior functional stability in culture over existing cell lines and primary cells.
Further, because iPSCs can be derived from adult donors via noninvasive methods (i.e., from a single peripheral blood draw), the potential exists to generate a panel of distinct hepatocyte cultures representing a series of defined genetic backgrounds for enabling the study of differential metabolic and toxin sensitivities as well as disease phenotypes. As such, the iCell Hepatocytes represent a new era for the study of liver function in vitro.
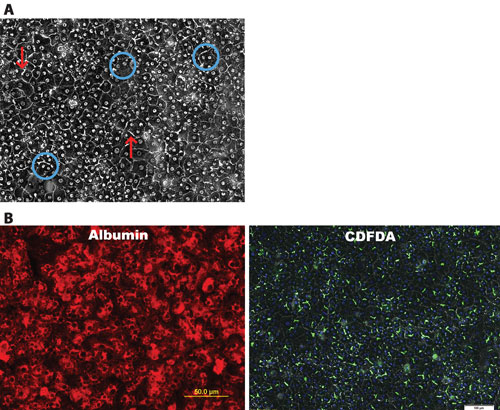
iCell Hepatocytes represent a highly pure (>95%) population of hepatocytes that demonstrate key morphological, phenotypic, and marker expression characteristics of those typically observed from primary tissue isolates (Figure 1).
These traits include a cobblestone appearance when plated in 2D culture as well as evidence by phase contrast microscopy of bi-nucleation and the formation of bile canaliculi. In addition, immunostaining illustrates the production of alpha-1-antitypsin (AAT) and albumin in the differentiated cells at levels suggestive of a highly pure culture. PAS and CDFDA staining further highlight the glycogen storage function and the formation of tight junctions and bile canaliculi in culture.
The failure of drug candidates in both the preclinical and clinical development phases of pharmaceutical research can be extremely costly and also have significant negative outcomes for patient health. The ability to accurately identify molecular liabilities of drug candidates, both in terms of metabolic susceptibility and/or hepatotoxic effects, early in development would significantly benefit the pharmaceutical discovery process. Thus researchers would be enabled to triage and deprioritize molecules with significant, predicted in vivo limitations from development while focusing on safer and more in vivo-stable therapeutics.
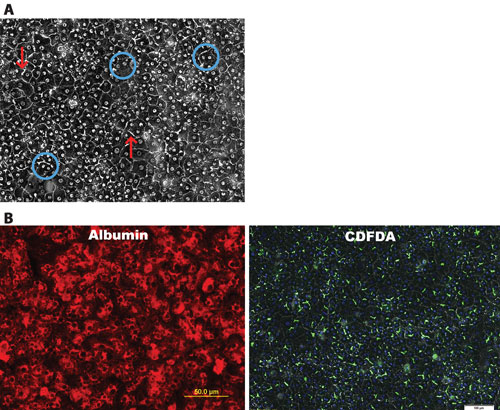
Figure 1. Key morphological features and expression of hepatocyte-specific markers are observed in iCell Hepatocytes: (A) Formation of an adherent monolayer, round nucleus, distinct nucleoli, high cytoplasmic/nuclear ratio as well as evidence of bi-nucleation (circles) and bile canaliculi (arrows). (B) Fluorescence microscopy shows evidence of purity by Albumin staining. 5-(6)-carboxy-2’,7’-dichlorofluorescein diacetate (CDFDA) staining highlights canalicular formation.
In addition, predictive toxicity models could also identify patient genotypes that should be excluded from specific clinical treatment groups if there is evidence of a population that could be particularly sensitive to an agent and have the potential to exhibit drug-induced liver injury (DILI) or even idiosyncratic toxicity.
As an exploration of the utility of human iPSC-derived hepatocytes as a predictive in vitro model for the study of hepatotoxicity, iCell Hepatocytes have been characterized for sensitivity to numerous known toxic agents and demonstrate a dose-dependent sensitivity to a variety of chemistries, including acetaminophen, troglitazone, terfenadine, tamoxifen, and chlorprimazine (data not shown). Further, the iPSC-differentiated hepatocytes exhibit behavior characteristic of metabolism-mediated toxin activation and sensitivity (Figure 2).
In the example shown, aflatoxin-B1 sensitivity is similar for iCell Hepatocytes and primary human hepatocytes in culture. However, the cancer cell-derived HepG2 line does not exhibit sensitivity to aflatoxin at 24 hours of exposure. Further, the addition of cytochrome P450 (CYP) inhibitors reduces the sensitivity of iPSC-differentiated hepatocytes to aflatoxin. Taken together, these data are consistent with a metabolically activated, reactive toxic form of aflatoxin being generated in the cultures and leading to cell death.
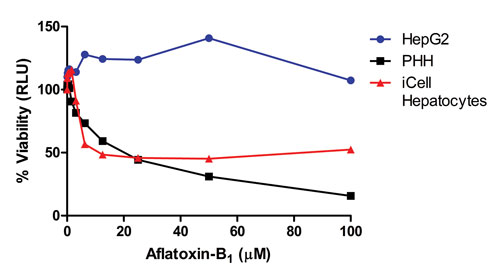
Figure 2. iCell Hepatocytes are sensitive to aflatoxin-B1, demonstrating metabolic function of the cells: A comparison of the sensitivity of three hepatocyte culture models to aflatoxin as measured by ATP production with the CellTiter-Glo® Assay (Promega) in the presence of compound. Similar sensitivities were determined for iCell Hepatocytes and primary human hepatocytes (PHH), but the hepatocellular carcinoma derived HepG2 cell line does not respond to aflatoxin over the concentration range and 24-hour exposure time tested. Data shown are courtesy of Promega.
Hepatitis C
Hepatitis viral infections, both HCV and HBV, remain common diseases in both the developing and developed worlds. These infections can lead to liver damage manifesting as fibrotic and cirrhotic liver tissue and can result in the need for liver transplantation. To date, cell-based models of HCV and HBV infection and the viral replication cycle have been limited.
In recent studies, we have observed the presence of markers required to afford HCV attachment to, and uptake by, iPSC-differentiated hepatocytes (Figure 3). iCell Hepatocytes have been observed to exhibit SR-B1 and CD81 proteins, which are required for viral attachment. In addition, the cells stain positively for Occludin and Claudin1, which are required for viral internalization within the cell.
The data shown demonstrate that iCell Hepatocytes are capable of HCV uptake as evidenced by the staining with a luciferase-tagged viral particle. Notably, virus uptake is inhibited in a dose-dependent fashion by the anti-CD81 blocking antibody. The use of human iPSC-derived hepatocytes for the study of HCV activity, as well as for the identification of novel therapeutics to prevent viral injury, are thus enabled by this system.
In summary, CDI’s iCell Hepatocytes are a predictive in vitro model system for the study of hepatotoxicity and metabolic function as well as disease modeling, including HCV studies. These human iPSC-derived hepatocytes provide an improvement over existing models by delivering a consistent, reproducible, and limitless source of liver tissue that is reflective of native human liver function.
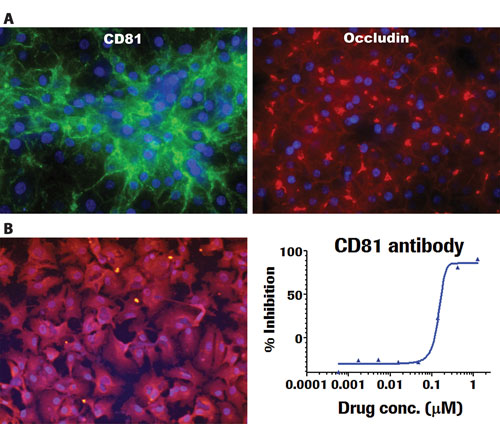
Figure 3. iCell Hepatocytes demonstrate key functional markers and responsiveness to HCV. (A) Immunostaining shows evidence of markers reported as key for cell attachment (CD81) as well as uptake (Occludin) of HCV. (B) iCell Hepatocytes are capable of HCV uptake as evidenced by staining with a luciferase-tagged viral particle. Virus uptake is inhibited in a dose dependent fashion by the anti-CD81 blocking antibody. Data are courtesy of Hoffmann-La Roche.
David A. Mann, Ph.D. ([email protected]), is iCell Hepatocytes product manager, Shannon Einhorn is technical application scientist II, Kristin Block is research specialist III, Peter Fuhrken, Ph.D., is group leader, and Christian Kannemeier, Ph.D., and Jen Luebke-Wheeler, Ph.D., are senior scientists, at Cellular Dynamics International.