Pancreatic β cells are not all alike, but β cell subtypes tend to be overlooked, mainly because it is difficult to distinguish between them. Although the blurring of β cells is understandable, it is still unfortunate. It retards research that could lead to regenerative therapies for diabetes.
Fortunately, recent studies are highlighting new ways to explore β-cell heterogeneity. Two such studies were cited in a “News and Views” article (“Physiology: Pancreatic β-Cell Heterogeneity Revisited”) that appeared July 11 in Nature. One of these studies was led by researchers based at Helmholtz Zentrum München and one by researchers based at the Oregon Stem Cell Center.
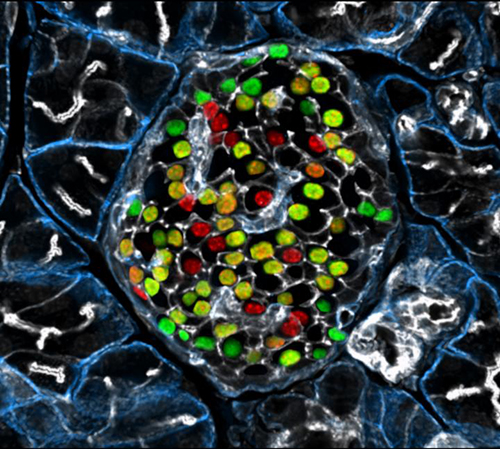
In the German study, researchers led by Professor Heiko Lickert searched for molecular markers subdividing the respective subgroups. One molecule, in particular, captivated their attention—the protein Flattop, which was present in about 80% of all β cells. These cells effectively determined the glucose concentration of their environment and secreted the corresponding amount of insulin, thus showing the metabolic properties of mature β cells.
Conversely, the team of researchers observed that β cells in which no Flattop was measurable showed a particularly high rate of proliferation. “In our experimental model, these cells proliferated up to four times more often than the Flattop-positive cells,” said Professor Lickert.
The Flattop findings appeared July 11 in Nature, in an article entitled, “Identification of Proliferative and Mature β-Cells in the Islets of Langerhans.”
“Here we show that Fltp (also known as Flattop and Cfap126), a Wnt/planar cell polarity (PCP) effector and reporter gene, acts as a marker gene that subdivides endocrine cells into two subpopulations and distinguishes proliferation-competent from mature β-cells with distinct molecular, physiological and ultrastructural features,” wrote the article’s authors. “Genetic lineage tracing revealed that endocrine subpopulations from Fltp-negative and -positive lineages react differently to physiological and pathological changes.”
“We show that 3D architecture and Wnt/PCP ligands are sufficient to trigger β-cell maturation,” the authors continued. “By contrast, the Wnt/PCP effector Fltp is not necessary for β-cell development, proliferation or maturation.”
Reflecting on the significance of these findings, Professor Lickert noted that Flattop-negative cells may constitute a kind of immature reserve pool, one that constantly renews itself and can replenish the mature β cells. Professor Lickert also indicated the importance of being able to subdivide β-cell subgroups; such a capability would allow a comprehensive analysis of the signaling pathways involved.
These results, obtained in studies in mice, raise hopes for the development of regenerative therapies. “The heterogeneity of the β cells has been studied for more than 50 years, now with enabling technologies it looks like we are beginning to understand how the cells behave,” observed Professor Lickert.
The American study provides further evidence for the existence of beta cell heterogeneity. It appeared July 11 in Nature Communications, in an article entitled, “Human Islets Contain Four Distinct Subtypes of β Cells.”
“Here we identify four antigenically distinct subtypes of human β cells, which we refer to as β1–4, and which are distinguished by differential expression of ST8SIA1 and CD9,” wrote the article’s authors. “These subpopulations are always present in normal adult islets and have diverse gene expression profiles and distinct basal and glucose-stimulated insulin secretion.”
The authors added that the β-cell subtype distribution is profoundly altered in type 2 diabetes. They also asserted that their findings suggest that “antigenically defined β-cell heterogeneity” is functionally and likely medically relevant.