GEN Magazine
Past Issues
Filter By Year
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
No Results
✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖![]()
✖

✖

✖

✖

✖

✖

✖![]()
✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖![]()
✖![]()
✖

✖![]()
✖

✖

✖![]()
✖

✖![]()
✖

✖![]()
✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖![]()
✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

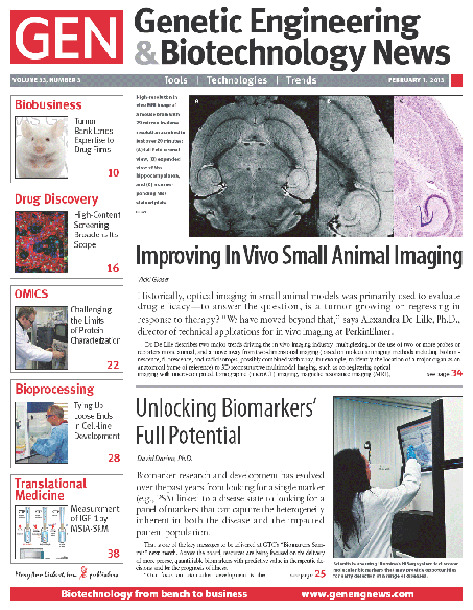
✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

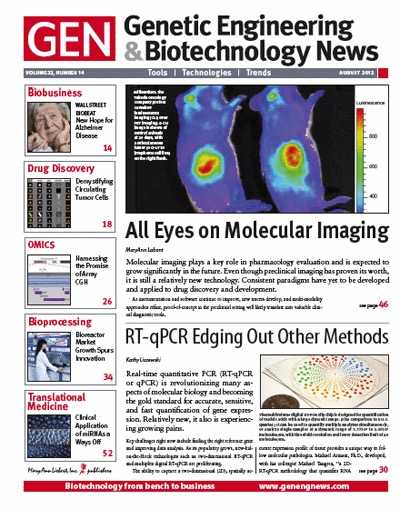
✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖

✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
✖![]()
