Doug Auld, Ph.D. Novartis Institutes for BioMedical Research
Researchers propose a fluorescent probe for adenosine triphosphate assay and describe a synthesis of a chromogenic cephalosporin for β-lactamase assay.
Bioluminescent methods exist to detect ATP that require coupling to firefly luciferase, an ATP-dependent enzyme. The assay described in the first article* uses a molecular beacon approach and an ATP-dependent DNA ligase. The ligase reaction proceeds through three steps: adenylation of an active site residue of the ligase to yield an enzyme-bound AMP with release of pyrophosphate, transfer of the AMP to the 5′-end of an oligonucleotide to yield a pyrophosphate bond, and formation of the 5′–3′ phosphodiester bond in the DNA.
In the assay, two oligonucleotides are hybridized to a “smart probe,” which is a DNA hairpin containing both a fluorophore and quencher at its ends (see Figure 1). Hybridization of the two oligonucleotides does not result in hairpin disruption, but upon ligation the resulting longer oligonucleotide opens the hairpin and relieves the quenching of the fluorophore (see Figure 1). The detection limit was found to be 0.5 nM (3 SD over background). This sensitivity is approximately one order of magnitude higher than bioluminescent approaches.
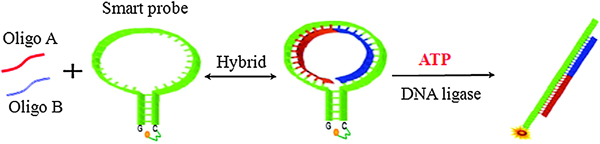
Figure 1. Schematic of real-time monitoring of ATP based on DNA ligation using smart probe. The oligo A and oligo B hybridize with the smart probe. In the presence of ATP, T4 DNA ligase catalyzes the DNA ligation of oligo A and oligo B. The ligation product then hybridizes with the smart probe, which restores the quenched fluorescence of the smart probe.
The second article by Yu et al.** describes the synthesis of a new chromogenic substrate for β-lactamase (bla). The bla enzyme is a commonly used reporter in cell-based assays in which a fluorescence-resonance-energy-transfer (FRET) substrate is used to measure activity.
The FRET substrate CCF2, consisting of a cephalosporin core linking 7-hydroxycoumarin to a fluorescein moiety, results in blue fluorescence when the substrate is cleaved by bla and green fluorescence when uncleaved, therefore a ratiometric assay is enabled using the blue/green fluorescence value. However, CCF2 is expensive as is Nitrocefin (the most commonly used absorbance-based substrate) and therefore the authors sought to synthesize improved absorbance-based substrates.
The authors describe a two-step synthesis to synthesize a chromogenic cephalosporin-[3-(4-nitrostyryl)-7-(2-phenylacetamido)-ceph-3-em-4-carboxylicacid (Chromacef). Cleavage of the β-lactam ring by bla results in a bathochromic shift of 64 nm (λmax from 378 nm to 442 nm; see Figure 2). The KM value for Chromacef using VIM-2 metallo-β-lactamase was 30 ± 7 μM and for TEM-1 it was 51 ± 15 μM, which are values similar to that obtained for Nitrocefin. The kcat values were improved compared to Nitrocefin.
Chromacef was shown to be cleaved by a variety of β-lactamases and should be a useful substrate to develop bla assays as well as serve as an orthogonal substrate.
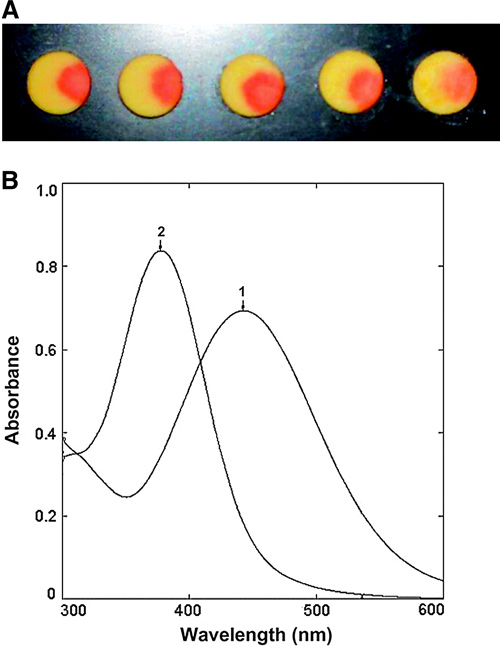
Figure 2. Chromacef color change on hydrolysis. (A) Color change on enzyme catalyzed hydrolysis of compound 6 by five different ß-lactamases. From left to right: Sigma-Aldrich type IV ß-lactamase from Enterobacter cloacae, KPC-1, DHA-1, VIM-2, and TEM-1. Each disk contained 50 lg of 6. (B) UV–visible spectra: spectrum 1 (λmax = 442) after enzymatic hydrolysis; spectrum 2 (λmax = 378 nm) unhydrolyzed Chromacef.
*Abstract from Analytical Biochemistry 2012, Vol. 429: 8–10
A novel fluorescent probe for adenosine triphosphate (ATP) assay based on DNA ligation is proposed in this article. This approach uses a novel smart probe, T4 DNA ligase, and two short oligonucleotides. In the presence of ATP, the T4 DNA ligase catalyzes the ligation reaction and the ligation product restores the fluorescence of the smart probe. This method is very sensitive with a 0.5-nM limit of detection. Compared with current assay methods, the strategy is simpler, cheaper, and 40 times more sensitive.
**Abstract from Analytical Biochemistry 2012, Vol. 428: 96–98
Production of β-lactamases is the primary mechanism of antibiotic resistance employed by gram-negative pathogens. Chromogenic β-lactams are important reagents for detection and assay of β-lactamases, but limited commercial availability and exorbitant pricing of these compounds are prohibitive. Here we describe a straightforward synthesis of a chromogenic cephalosporin for β-lactamase assay that gives an overall yield of 74%. On hydrolysis, its λmax undergoes a bathochromic shift that is easy to see and measure spectrophotometrically with a Δ442 nm of 14,500 cm−1·M−1. This compound was shown to be a substrate for a variety of β-lactamases.
Doug Auld, Ph.D., is affiliated with the Novartis Institutes for BioMedical Research.
ASSAY & Drug Development Technologies, published by Mary Ann Liebert, Inc., offers a unique combination of original research and reports on the techniques and tools being used in cutting-edge drug development. The journal includes a “Literature Search and Review” column that identifies published papers of note and discusses their importance. GEN presents here one article that was analyzed in the “Literature Search and Review” column on two papers published in Analytical Biochemistry: “Fluorescence detection of adenosine triphosphate using smart probe,” authored by Ma C, Chen H, Han R, He H, and Zeng W; and “A chromogenic cephalosporin for β-lactamase inhibitor screening assays,” authored by Yu S, Vosbeek A, Corbella K, Severson J, Schesser J, and Sutton LD.