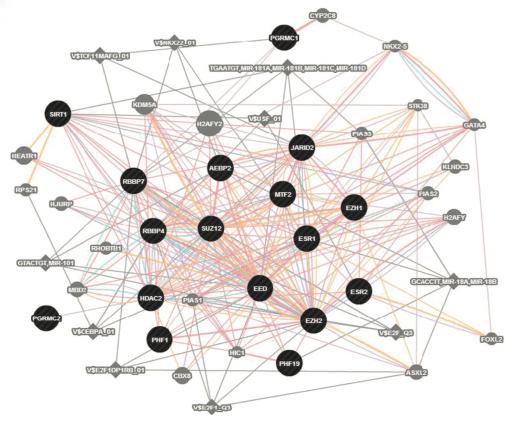
![The expression of the Extra Sex Combs/Enhancer of Zeste, or ESC/E(Z), gene network was found to be systematically disturbed in PMDD. [Peter Schmidt, M.D./NIMH; David Goldman, M.D./NIAAA]](https://genengnews.com/wp-content/uploads/2018/08/Jan3_2017_SchmidtGoldman_PMDD2516771432-1.jpg)
The expression of the Extra Sex Combs/Enhancer of Zeste, or ESC/E(Z), gene network was found to be systematically disturbed in PMDD. [Peter Schmidt, M.D./NIMH; David Goldman, M.D./NIAAA]
Even though its causes have been unclear, premenstrual dysphoric disorder (PMDD) has been described as a severe form of premenstrual syndrome (PMS). Affecting 2–5% of women of reproductive age, PMDD is like PMS in that it follows a predictable, cyclic pattern. Yet PMDD, which is marked by disabling irritability, sadness, and anxiety, is associated with unusual sensitivity to fluctuations in sex hormones. This sensitivity has motivated researchers to focus on PMDD’s potential biological drivers, rather than on psychological or cultural phenomena.
New results from scientists based at the National Institutes of Health (NIH) suggest that PMDD arises from certain molecular mechanisms. These scientists report that they have found that a large gene complex shows a conspicuous difference in its response to ovarian steroids.
“We found dysregulated expression in a suspect gene complex which adds to evidence that PMDD is a disorder of cellular response to estrogen and progesterone,” asserted Peter Schmidt, M.D. of the NIH's National Institute of Mental Health, Behavioral Endocrinology Branch. “Learning more about the role of this gene complex holds hope for improved treatment of such prevalent reproductive endocrine-related mood disorders.”
The new findings appeared January 3 in the journal Molecular Psychiatry, in an article entitled, “The ESC/E(Z) Complex, an Effector of Response to Ovarian Steroids, Manifests an Intrinsic Difference in Cells from Women with Premenstrual Dysphoric Disorder.” The article described how the NIH team studied the genetic control of gene expression in cultured white blood cell lines from women with PMDD and controls. These cells express many of the same genes expressed in brain cells—potentially providing a window into genetically influenced differences in molecular responses to sex hormones.
“In this study, lymphoblastoid cell line cultures (LCLs) from women with PMDD and asymptomatic controls were compared via whole-transcriptome sequencing (RNA-seq) during untreated (ovarian steroid-free) conditions and following hormone treatment,” wrote the article’s authors. “The women with PMDD manifested ovarian steroid-triggered behavioral sensitivity during a hormone suppression and addback clinical trial, and controls did not, leading us to hypothesize that women with PMDD might differ in their cellular response to ovarian steroids.”
An analysis of all gene transcription in the cultured cell lines turned up a large gene complex in which gene expression differed conspicuously in cells from patients compared to controls. Notably, this ESC/E(Z) (Extra Sex Combs/Enhancer of Zeste) gene complex regulates epigenetic mechanisms that govern the transcription of genes into proteins in response to the environment—including sex hormones and stressors.
More than half of the ESC/E(Z) genes were overexpressed in PMDD patients' cells, compared to cells from controls. But paradoxically, protein expression of four key genes was decreased in cells from women with PMDD. In addition, progesterone boosted expression of several of these genes in controls, while estrogen decreased expression in cell lines derived from PMDD patients. This suggested dysregulated cellular response to the hormones in PMDD.
“These findings demonstrate that LCLs from women with PMDD manifest a cellular difference in ESC/E(Z) complex function both in the untreated condition and in response to ovarian hormones,” the article’s authors concluded. “Dysregulation of ESC/E(Z) complex function could contribute to PMDD.”
“For the first time, we now have cellular evidence of abnormal signaling in cells derived from women with PMDD, and a plausible biological cause for their abnormal behavioral sensitivity to estrogen and progesterone,” explained Dr. Schmidt.
Using cutting edge “disease in a dish” technologies, the researchers are now following up the leads discovered in blood cell lines in neurons induced from stem cells derived from the blood of PMDD patients—in hopes of gaining a more direct window into the ESC/E(Z) complex's role in the brain.