In Fantastic Voyage, a classic sci-fi film, a submarine was shrunk to nanoscale size and injected into a patient, who was ultimately saved by the submarine’s nanoscale crewmembers, who used a laser to obliterate a dangerous blood clot. Now, curiously, elements of that story are being replayed in real life, albeit in mice that are in need of chemotherapy. Also, the chemotherapy isn’t delivered by anything as flashy as a miniaturized submarine. Instead, the carriers are porphyrin-phospholipid liposomes, which are less formally known as nanoballoons.
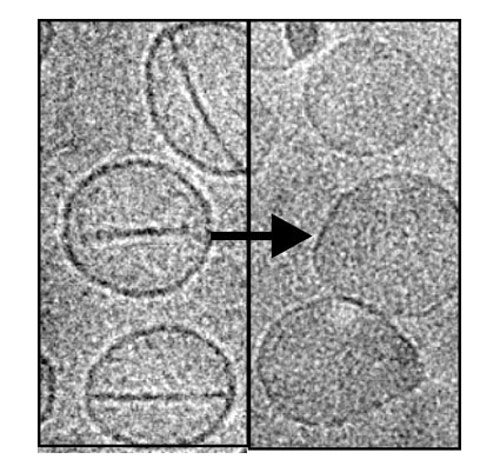
While the nanoballoons lack the sleek lines of the Proteus (the intrepid craft in the film), they have their own charms. They are, essentially, liposomes, which are artificial vesicles—bi-lipid-walled, sphere-shaped containers. Various kinds of liposomes have been developed to carry and deliver drugs, but they have had only mixed success. They struggle with physiological barriers, and they don’t always release their cargo when and where it is needed. To overcome these limitations, scientists have developed a new kind of liposome. As indicated above, it incorporates a porphyrin-phospholipid, or PoP.
PoP liposomes can be permeabilized by irradiation with near-infrared light. More to the point, when PoP liposomes are struck by a laser, they pop open—on demand—and release their cargo, which may consist of fluorophores, antibiotics, or chemotherapeutics. What's more, as soon as the laser is turned off, the PoP liposomes close, taking in proteins and molecules that might induce cancer growth. The PoP liposome’s developers foresee doctors using them to collect such tell-tale debris, which could be analyzed once the PoP liposomes are retrieved via blood draws or biopsies.
The PoP liposome was put through its paces in studies with mice, as described in an article that appeared April 3 in Nature Communications. These studies, led by Jonathan Lovell, Ph.D., assistant professor of biomedical engineering, University at Buffalo, produced these observations: “PoP liposomes demonstrate spatial control of release of entrapped gentamicin and temporal control of release of entrapped fluorophores following intratumoral injection. Following systemic administration, laser irradiation enhances deposition of actively loaded doxorubicin in mouse xenografts, enabling an effective single-treatment antitumor therapy.”
A more detailed excerpt reads as follows: “Mice were injected with doses of 10 mg kg–1 Dox-PoP liposomes. Fifteen minutes following i.v. injection, tumors were irradiated with 658 nm laser light at 200 mW cm–2 for 12.5 min (150 J cm–2), or were not irradiated as a control. Twenty-four hours later, organs were collected and Dox biodistribution was assessed. Laser treatment resulted in the deposition of about threefold more Dox compared with nonirradiated tumors, whereas none of the other organs displayed statistically significant differences.”
When describing how the PoP liposomes worked, Dr. Lovell actually used language that brought Fantastic Voyage to mind: “Think of it this way. The nanoballoon is a submarine. The drug is the cargo. We use a laser to open the submarine door, which releases the drug. We close the door by turning the laser off. We then retrieve the submarine as it circulates through the bloodstream.”
Dr. Lovell intends to continue fundamental studies to better understand why the treatment works so well in destroying tumors in mice, and to optimize the process. Human trials could start within five years, he said.