A weight-control mechanism has been detailed by scientists based at NGM Biopharmaceuticals, a company that is already exploiting its newfound knowledge. NGM Bio indicates that it is working with Merck to develop drug candidates for both excess and insufficient weight.
One of the drug candidates, NGM386, holds potential as a treatment for obesity. Another candidate, NGM120, holds potential as a treatment for cachexia, an extreme form of weight loss exacerbated by chemotherapy.
Each drug acts on a molecular pathway that is localized in brainstem neurons. These neurons, though found deep within the brain, far from areas of the brain normally associated with weight control, form a kind of “emergency circuit.” Ordinarily, this circuit is active only if the body experiences acute or prolonged stress. If, for example, the body is exposed to toxins, such as chemotherapeutic agents, or is burdened by chronic disease, such as cancer or obesity, the emergency circuit is triggered to balance food consumption and energy expenditure.
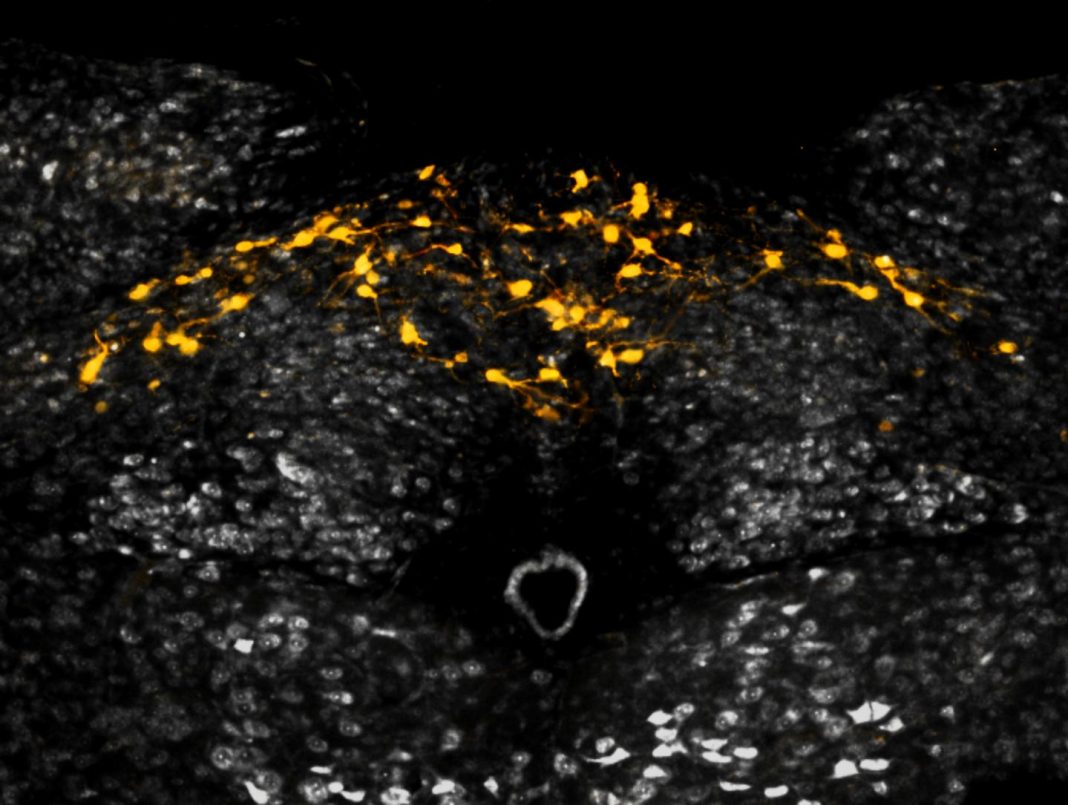
The trigger is a sort of toggle switch, and its main components are a little-understood hormone, growth and differentiation factor 15 (GDF15), and a brainstem-restricted receptor, glial cell-derived neurotrophic factor (GDNF) receptor α-like (GFRAL). How these components interact has been studied by NGM Bio scientists, who reported their latest findings September 27 in the journal Nature, in an article entitled “Non-Homeostatic Body Weight Regulation through a Brainstem-Restricted Receptor for GDF15.”
The paper shows that GDF15 binding to GFRAL is required both to protect mice from weight gain in metabolically stressed conditions and to trigger excessive weight loss in mice treated with chemotherapy. This work also demonstrated that GFRAL forms a complex with the receptor tyrosine kinase RET on the surface of neurons that are localized exclusively in the brainstem.
Essentially, the NGM Bio scientists described how GDF15 regulates food intake, energy expenditure, and body weight in response to metabolic and toxin-induced stresses.
“…we show that Gfral knockout mice are hyperphagic under stressed conditions and are resistant to chemotherapy-induced anorexia and body weight loss,” wrote the authors of the Nature article. “By isolating GFRAL as the receptor for GDF15-induced anorexia and weight loss, we identify a mechanistic basis for the non-homeostatic regulation of neural circuitry by a peripheral signal associated with tissue damage and stress.”
These findings, the authors emphasized, provide opportunities to develop therapeutic agents for the treatment of disorders with altered energy demand.
“While it had been established that GDF15 is involved in weight-related diseases, the lack of information about its signaling pathway had impeded the development of new medicines harnessing this biology,” said Jin-Long Chen, Ph.D., founder and chief scientific officer of NGM Bio. “This publication describes the critical breakthrough that enabled the development of multiple drug candidates for these difficult-to-treat disorders, which could ultimately transform the lives of patients battling obesity and cachexia.”
Until recently, the identity of circulating molecules that could activate the deep-brain emergency circuit remained elusive. The current study, however, adds to findings from several other recent studies, by showing that GDF15 may interact with receptors on the circuit’s neurons to regulate body weight. According to the current study, GDF binds to GFRAL and activates RET, thereby “flipping the switch” on the emergency circuit, leading to anorexia and weight loss.
“GDF15 activates GFRAL-expressing neurons localized exclusively in the area postrema and nucleus tractus solitarius of the mouse brainstem,” the Nature paper detailed. “It then triggers the activation of neurons localized within the parabrachial nucleus and central amygdala, which constitute part of the ‘emergency circuit’ that shapes feeding responses to stressful conditions. GDF15 levels increase in response to tissue stress and injury, and elevated levels are associated with body weight loss in numerous chronic human diseases.”
NGM's research supports the hypothesis that obesity and cachexia are opposite sides of the same biological process. Drugs such as NGM386 and NGM120 that can toggle the GFRAL “switch” on or off may have the potential to offer therapeutic benefit for a range of weight-related diseases.
“GFRAL was discovered to be the receptor for GDF15 by one of our dedicated scientists whose commitment to drug discovery prompted her to visit the lab on a holiday weekend to check on an experiment. In the ensuing years, working in collaboration with Merck, our team has advanced multiple product candidates against the GDF15 receptor complex for human testing,” related William J. Rieflin, CEO of NGM Bio. “We hope the dedication of our researchers and clinicians will ultimately translate into medicines for patients who struggle with weight-related diseases.”