Dr. Reddy discussing findings from new research on developing drugs toward RAS.
![Mechanism of new research targeting previously undruggable RAS gene. [Cell, Volume 165, 21 April 2016]](https://genengnews.com/wp-content/uploads/2018/08/fx111851571281-1.jpg)
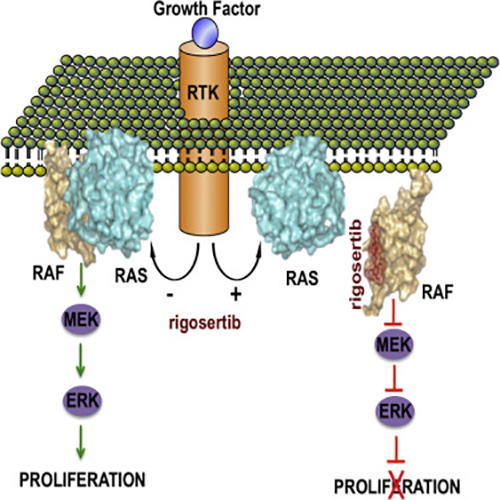
Mechanism of new research targeting previously undruggable RAS gene. [Cell, Volume 165, 21 April 2016]
For greater than 30% of human cancers, RAS genes are mutated and have been implicated as key tumor drivers—making them some of the most sought after cancer drug targets. However, the absence of any drug-binding pockets in the mutant RAS proteins has made drug development extremely difficult. Yet now, a new study from researchers at the Icahn School of Medicine at Mount Sinai has identified a new mechanism for targeting this important cancer gene.
Mutations in RAS genes—such as HRAS, KRAS, and NRAS—are frequently observed in many of the most common and lethal tumors, including cancers of the pancreas, lung, and colon. While scientists have made significant headway in understanding these mutations and their impact on cellular signaling, little headway has been made toward developing drugs that systematically target the RAS oncogenes. This lack of progress has led many in the field to label RAS an “undruggable” cancer gene.
The Mt. Sinai team identified what they believe to be the first small molecule able to simultaneously inhibit the different signaling pathways activated by RAS oncogenes. This compound called rigosertib or ON01910.Na, acts as a protein-protein interaction inhibitor that prevents binding between RAS and signaling proteins (including RAF, PI3K, and others) that drive a cell into a cancer cell.
“Here, we present evidence that rigosertib, a styryl-benzyl sulfone, acts as a RAS-mimetic and interacts with the RBDs of RAF kinases, resulting in their inability to bind to RAS, disruption of RAF activation, and inhibition of the RAS-RAF-MEK pathway,” the authors wrote. “We also find that rigosertib binds to the RBDs of Ral-GDS and PI3Ks. These results suggest that targeting of RBDs across multiple signaling pathways by rigosertib may represent an effective strategy for inactivation of RAS signaling.”
The findings from this study were published recently in Cell through an article entitled “A Small Molecule RAS-Mimetic Disrupts RAS Association with Effector Proteins to Block Signaling.”
Additionally, the investigators performed structural experiments to confirm the mode of action for rigosertib and also demonstrated the potential for this targeted mechanism in the treatment of several RAS-driven cancers.
“This discovery is a significant breakthrough for the cancer field,” explained senior study author E. Premkumar Reddy, Ph.D., professor of oncological sciences at the Icahn School of Medicine at Mount Sinai. “Rigosertib's mechanism of action represents a new paradigm for attacking the intractable RAS oncogenes. Our current focus is to use the information from our studies with rigosertib to design the next generation of small molecule RAS-targeting therapies, and we are excited to have recently identified several compounds which we think improve on the qualities of rigosertib.”