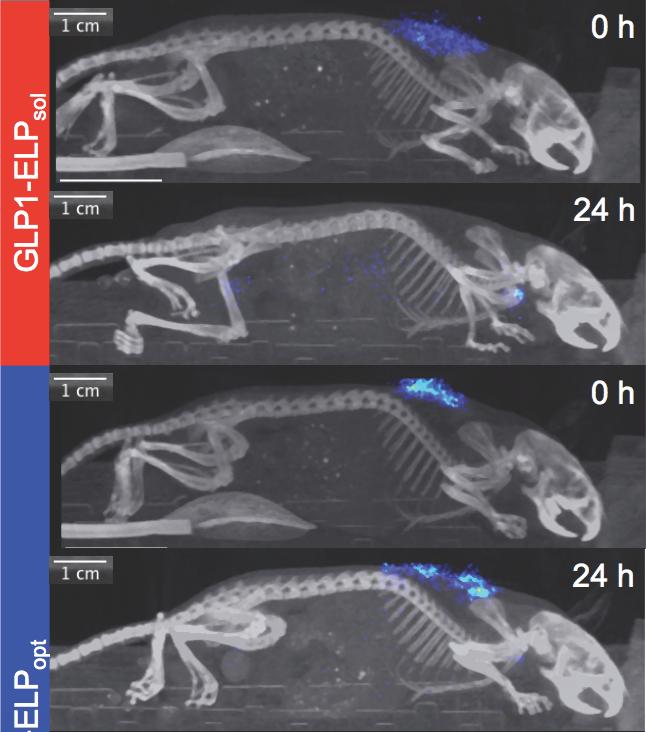
A new biopolymer-containing formulation may improve the delivery of glucose-control medication, extending the time a single injection stays effective. (Top two photos) A glucose-controlling drug (blue) is shown completely dissolving after 24 hours in the body of a mouse. (Bottom two photos) A newly optimized version of a diabetes treatment forms a “depot” for controlled release that persists more than 24 hours. [Ashutosh Chilkoti/Duke University]
The daily or once-weekly insulin shot—necessary for the control of type 2 diabetes—could be replaced by a twice- or even once-a-month shot. A new, longer-lasting injectable formulation has been developed that combines a familiar diabetes-control molecule, glucagon-like peptide-1 (GLP1), with a heat-sensitive elastin-like polypeptide (ELP). Once a solution containing the GLP1–ELP combo passes through a standard needle and penetrates the skin, it reacts to body heat, forming a biodegradable gel-like “depot” that slowly releases the drug as it dissolves.
The novel drug-delivery mechanism was developed by scientists based at Duke University, who assert that it could be used to “enhance therapeutic outcomes by eliminating peak-and-valley pharmacokinetics and improving overall safety and tolerability.” The scientists, led by Ashutosh Chilkoti, Ph.D., chair of the department of biomedical engineering at Duke, suggest that their work could be “broadly applicable”; that is, it could improve the pharmacological performance of peptides and protein therapeutics besides GLP1.
Details of the work appeared June 5 in the journal Nature Biomedical Engineering, in an article entitled “One-Week Glucose Control via Zero-Order Release Kinetics from an Injectable Depot of Glucagon-Like Peptide-1 Fused to a Thermosensitive Biopolymer.” The “one week” indicated in the title refers to the drug depot’s performance in mice. Glucose control, the scientists found, was more durable in rhesus monkeys, and even longer glucose control, the scientists suggested, could be achieved in humans, since humans have slower metabolisms than mice or monkeys.
“A subcutaneous depot formed after a single injection of GLP1 recombinantly fused to a thermosensitive elastin-like polypeptide results in zero-order release kinetics and circulation times of up to 10 days in mice and 17 days in monkeys,” the authors of the article indicated. “The optimized pharmacokinetics lead to 10 days of glycaemic control in three different mouse models of diabetes, as well as the reduction of glycosylated haemoglobin levels and weight gain in ob/ob mice treated once weekly for 8 weeks.”
Many current treatments for type 2 diabetes use GLP1, a signaling molecule that causes the pancreas to release insulin to control blood sugar. However, this peptide has a short half-life and is cleared from the body quickly.
To make treatments last longer, researchers have previously fused GLP1 with synthetic microspheres and biomolecules like antibodies, making them active for 2 to 3 days in mice and up to a week in humans. Currently, the longest-acting glucose control treatment on the market, dulaglutide, requires a once-weekly injection, while standard insulin therapies often have to be injected twice or more every day. Despite improvements such as these, many treatments don't include a mechanism to control the rate of the peptide's release, and treatment effectiveness can plateau after prolonged use.
The Duke researchers persisted with their ongoing experiments, which focused on thermosensitive delivery biopolymers. By varying the design of their delivery biopolymers at the molecular level, they found a “sweet spot” that maximized the duration of the drug's delivery from a single injection, noted Dr. Chilkoti. “By doing so,” he continued, “we managed to triple the duration of this short-acting drug for type 2 diabetes, outperforming other competing designs.”
Building upon their previous work with the drug and delivery system, researchers in the Dr. Chilkoti’s laboratory optimized their solution to regulate glucose levels in mice for 10 days after a single injection, up from the previous standard of 2 to 3 days.
In further tests, the Duke team found that the optimized formulation improved glucose control in rhesus monkeys for more than 14 days after a single injection, while also releasing the drug at a constant rate for the duration of the trial.
“What's exciting about this work was our ability to demonstrate that the drug could last over 2 weeks in nonhuman primates,” remarked Kelli Luginbuhl, a Ph.D. student in Dr. Chilkoti’s laboratory and co-author of the study. “Because our metabolism is slower than monkeys and mice, the treatment should theoretically last even longer in humans, so our hope is that this will be the first biweekly or once-a-month formulation for people with type 2 diabetes.”
Despite a variety of treatment options, managing type 2 diabetes still poses a problem. Patients don't always reach their glycemic targets, and adherence to a treatment plan that relies on frequent, meal-specific dosing leaves room for human error. By limiting the number of injections a person will need to control their glucose levels, the researchers hope this new tool will improve treatment options for the disease.
The researchers now plan to study the immune response to repeated injections and test the material with other animal models. They are also considering additional applications for this controlled-release system, such as delivering pain medication.
Dr. Chilkoti also indicated that because the drug is synthesized inside Escherichia coli bacterial cultures instead of mammalian cells, it is cheaper and faster to produce, making it a potential target for use in developing countries once it's commercialized.
According to a report issued last year by Grand View Research, the global insulin market is expected to reach $53.04 billion by 2022. Grand View anticipates that the most lucrative segment will consist of long-acting analogs. The segment’s high growth rate, estimated at 15.0%/year, is accounted for by fast-selling products such as Lantus by Sanofi Aventis. Moreover, the addition of new products such as Novo Nordisk’s Tresiba ultra-long-acting analog is expected to further drive segment growth. Tresiba is administered subcutaneously once daily at any time of day. Even longer-lasting formulations, such as those contemplated by Dr. Chilkoti’s team, may contribute to yet more growth in the segment.


