Liquid biopsy is a new technology that analyzes nonsolid biological tissues, such as blood, and it is less invasive and more sensitive than traditional biopsies. Liquid biopsies can be used for a broad range of samples, such as circulating tumor cells (CTCs), circulating cell-free DNA (cfDNA), cell-free microRNA, exosomes, and blood platelets, and are widely used in exploring the molecular landscapes of tumors. Compared with tissue-based profiles, liquid biopsy can provide time sequenced picture rather than a snapshot of tumor heterogeneity for the samples that can be obtained repeatedly. For such reasons, liquid biopsy is suitable for longitudinal surveillance of patients and evaluation of prognosis to guide patient care and be integrated into clinical practice (Siravegna et al., 2017). As a new type of liquid biopsy, CTCs are a population of cells shed from a primary or metastatic tumor into the peripheral blood and lymph (Pantel et al., 2009; Yu et al., 2011). Analysis of CTCs through next-generation sequencing (NGS) and whole genome amplification (WGA) can reveal the mechanism of tumor metastasis, intra-tumor heterogeneity, and genetic alterations. In recent years, CTCs have exhibited great potential for noninvasive diagnosis and real-time monitoring of cancer.
Single-cell sequencing enables researchers to recognize a problem from a new perspective and provides a new method to identify etiology at the genomic level. Analysis of CTCs using single-cell sequencing can help to reveal the underlying mechanisms of tumorigenesis and metastasis and to identify gene mutations that potentially contribute to tumor metastasis or drug resistance. Through identifying specific mutations and markers in CTCs, individualized therapy may be formulated, thus improving understanding of the molecular mechanisms of tumor metastasis, relapse, and chemotherapy resistance.CTCs and Tumor Metastasis
CTCs are a population of cells that are shed from primary or metastatic tumor deposits and migrate into peripheral blood. CTCs are related to tumor metastasis (Pantel et al., 2009; Yu et al., 2011), which is considered to be the major cause of cancer-associated mortality (Figure 1). CTCs are predictive biomarkers in clinical care, owing to their close relationship with tumor metastasis (Aceto et al., 2014). Notably, CTCs have been found to appear in vasculature before detection of the primary tumor (Kalluri and Weinberg, 2009). Tumor cell metastasis is a complex process consisting of a series of stages that include the loss of adhesion proteins (e.g., E-cadherin), shedding of tumor cells, intravasation, dissemination, arrest at secondary sites, extravasation, colonization, and formation of metastatic tumors (Chaffer and Weinberg, 2011; Gradilone et al., 2011; Krebs et al., 2014). During this process, two crucial changes typically occur in CTCs: epithelial to mesenchymal transition (EMT) and mesenchymal to epithelial transition (MET). EMT is a highly complicated process in which cancer cells revert to a state resembling the mobile cells in the developing embryo (Hanahan and Weinberg, 2011). MET usually occurs when CTCs arrest at the primary tumor or other metastatic lesion site and become arrested in a state known as dormancy or undergo alterations to adjust to the new environment. Whereas EMT has been demonstrated in human cancer cells in the circulation, the requirement for EMT to initiate metastasis remains a debated topic (Ledford, 2011; Yu et al., 2013).
CTC Detection and Isolation
In recent years, CTCs have exhibited potential for tumor diagnosis, treatment, and monitoring, and great efforts have been made to detect and separate CTCs. Along with advancements in CTC enrichment techniques, the efficacy and accuracy of CTC capture have greatly improved. CTC isolation techniques can be classified into three types: nucleic acid-based, physical property-based and surface marker-based methods (Figure 2). Each method has specific characteristics, application range, and limitations, which are listed below.
Nucleic acid-based methods
Nucleic acid-based CTC detection identifies specific tumor DNA or mRNA (cfDNA) to confirm the presence of CTCs indirectly (Esmaeilsabzali et al., 2013). Detection involves designing specific primers to combine with specific DNA or cDNA sequences that are extracted from enriched samples. These genes represent specific tumor genes that contain known mutations, translocations, and methylation patterns (Riethdorf et al., 2008). The nucleic acid-based method has the highest sensitivity but lacks specificity, owing to the potential of captured noncancerous cells to generate false-positive signals, thus decreasing the overall accuracy. According to previous work, not all the detection targets are tumor specific, because the same targets can be found in blood cells (Fehm et al., 2009; Punnoose et al., 2010). Pantel et al. (2008) have reported that CK-19, a major marker for CTC detection, is also presenting immune cells.
Physical property-based methods
Physical property-based methods isolate CTCs on the basis of physical properties, including size, density, mechanical plasticity, and dielectric properties, and have the advantage of allowing CTC separation without marker-based preselection. CTCs (20–30 μm) are larger than most blood cells (8–12 μm), and thus, they can be isolated through specific porous filters that allow only blood cells to cross. This technique has been used in commercialized ISET® (Rarecells, Paris, France) and ScreenCell® systems (ScreenCell, Paris, France) for isolation of fixed and live CTCs (Desitter et al., 2011). Owing to their relatively larger size and larger mass than those of blood cells, CTCs can also be separated using density gradient centrifugation. In addition to size and density disparity, differences in mechanical properties between blood cells and CTCs have been exploited to capture CTCs; specifically, blood cells have inherent deformability and are smaller, whereas CTCs are larger and stiffer (Yap and Kamm, 2005). On the basis of different cells have different conductivities and polarities, CTCs can be isolated on the basis of their unique dielectric properties when they are placed in an electric field (Kobayashi et al., 2015). Nevertheless, physical property-based CTC detection is subject to the physical properties of tumor cells, and some immune cells (monocytes) are similar in size to certain types of tumor cells.
Surface marker-based methods
In comparison to the above reported methods, the surface marker-based method has been widely used to capture CTCs. There are a variety of applications of this principle in commercially available products, such as the CellSearch®System, autoMACS® Pro separator, and IsoFlux™ systems. Two general approaches have been developed: positive selection and negative selection. In positive selection, CTC-specific cell surface markers are used to purify and separate CTCs from normal blood cells, whereas in negative selection, leukocyte-specific cell surface markers are applied to remove immune cells from the blood. The most frequently used method of positive selection is utilizing epithelial cell adhesion molecule (EpCAM), which is consistently expressed by epithelial-derived tumor cells but cannot be found on normal leukocytes. Size-dictated immunocapture chip (SDI-Chip) is a microfluidic chip that captures CTCs based on surface marker and realizes a capture efficiency greater than 92% with a purity of 82% in nonmetastasis colorectal patients. More importantly, this method also unites several physical factors, such as frequent interactions of cells with the immunocoated surface, extended duration of contact to assure bond formation, and reasonable hydrodynamic forces, which improve the accuracy and purity to some extent. Negative selection is applied when CTCs express low levels of EpCAM, particularly cells that undergo EMT, by depleting normal hematopoietic cells from blood samples, thus leaving the CTCs (Gorges et al., 2012; Yu et al., 2013).

Single CTC Sequencing and Analysis
The first and only clinically validated, FDA-approved test for capturing and enumerating CTCs to aid in decision-making with regard to clinical care for metastatic breast, prostate, and colorectal cancer patients is the CellSearch System. Evaluation of CTCs allows for assessment of patient prognosis and is predictive of progression-free survival and overall survival (Coumans and Terstappen, 2015). However, the limitations of CellSearch are that CTC detection in the blood is based on epithelial biomarkers, which may be downregulated or not expressed in some tumor types (Joosse and Pantel, 2013), and that the evaluation of prognosis simply depends on cell count and does not take individual CTC characteristics or patients’ genetic characteristics into account. Therefore, appropriate treatment cannot be achieved. Single-cell sequencing enables researchers to recognize the problem from a new perspective and provides a new method to identify etiology at the genomic level. Through identifying specific mutations and markers in CTCs, individualized therapy could potentially be formulated by doctors. This information would also help researchers better understand the molecular mechanisms underlying tumor metastasis, relapse, and chemoresistance and might eventually aid in the development of new targeted cancer therapies.
After isolation of CTCs, single-cell sequencing should be performed to identify genomic and transcriptomic characteristics of CTCs. The key to obtaining accurate information about CTCs is to amplify DNA or cDNA in individual CTCs from picogram to microgram amounts to satisfy the requirements of NGS (Van Loo and Voet, 2014). The two genome amplification technologies that have been widely used for this purpose are multiple displacement amplification (MDA) (Dean et al., 2002) and polymerase chain reaction (PCR) (Cheung and Nelson, 1996) or a combination of the two. Both technologies bring about technical artifacts derived from nonuniform genome coverage, such as biased amplification of sequences rich in cytosine and guanosine (GC-bias), preferential allelic amplification or allelic dropout, base copy errors, and chimeric DNA molecules (Macaulay and Voet, 2014). Multiple annealing and loop-based amplification cycles (MALBAC), a technique that can achieve broad coverage of the genome together with uniform amplification and a substantially lower allele dropout rate in comparison to MDA, combines preamplification by a polymerase with strand displacement activity and amplification through PCR and is widely used for simultaneous characterization of single nucleotide polymorphisms and copy number variations (CNVs) (Zong et al., 2012; Leung et al., 2015).
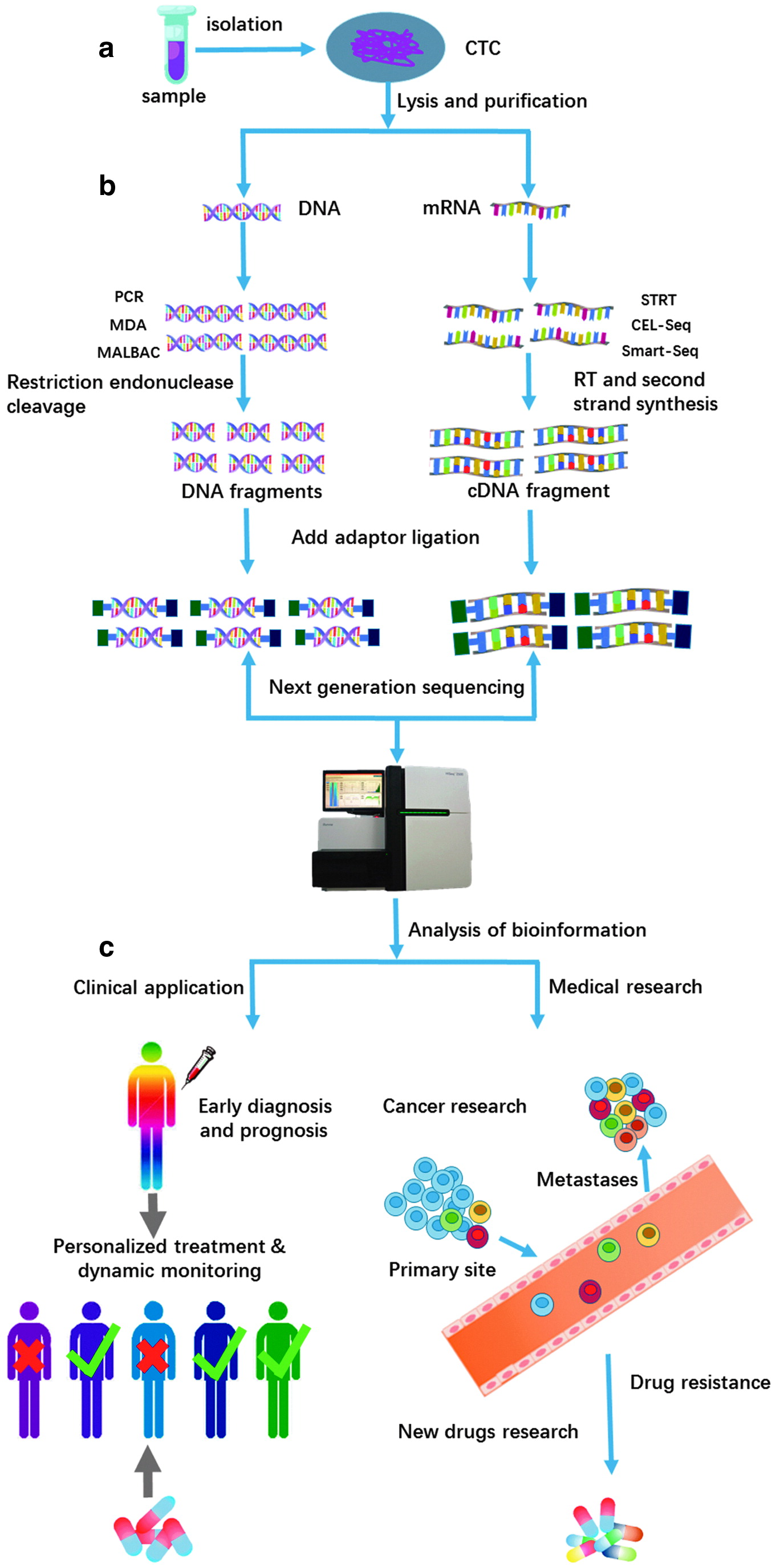
Single-cell transcriptome sequencing can measure the distribution of gene expression levels in individual cells. Common single-cell transcriptome sequencing techniques include Smart-seq2 (Ramskold et al., 2012), STRT (Islam et al., 2011), and CEL-seq (Hashimshony et al., 2012). The basic steps of transcriptome sequencing are synthesis of complementary single DNA strands through reverse transcription and PCR amplification with or without a template switch and sequencing through NGS. CEL-seq and STRT-seq integrate a barcode comprising a stretch of eight nucleotides that uniquely label all mRNAs from the same cell into the sequencing primer. However, these two methods cannot achieve complete read coverage and cause amplification bias in different regions of mRNA molecules. Compared with these two methods, Smart-seq2 methods are a more recent alternative to obtain read coverage along the entire transcript (Picelli et al., 2013). Apart from the read coverage rate, increasing throughput, lowering the cost of single-cell sequencing, and improving sequencing sensitivity must be taken into account. Four advanced droplet-based microfluidic methods, Drop-seq and inDrop sequencing, SDI-Chip, and Seq-Well, have recently been reported and can dramatically increase the throughput to thousands of cells while simultaneously minimizing the sequencing costs (Klein et al., 2015; Macosko et al., 2015; Ahmed et al., 2017; Gierahn et al., 2017). External spike-in of RNA of known concentration is a method to quantify sensitivity (Baker et al., 2005; Hashimshony et al., 2012) (Figure 3). Among the four technologies, Seq-Well realizes a portable, low-cost, and massively-parallel single cell RNA-seq platform. By combining bar-coded mRNA capture beads and semipermeable membrane and sealing single cell in an array of subnanoliter wells, Seq-Well enables higher capture efficiency and throughput. Moreover, this device is inexpensive and user-friendly, which reduces the cost of research and is expected to large-scale application (Gierahn et al., 2017).
For this article in its entirety with references click here.
DNA and Cell Biology, published by Mary Ann Liebert, Inc., is the trusted source for authoritative, peer-reviewed reporting on the latest research in the field of molecular biology. The above article was first published in the February 2018 issue of DNA and Cell Biology with the title “Clinical Applications of and Challenges in Single-Cell Analysis of Circulating Tumor Cells“. The views expressed here are those of the authors and are not necessarily those of DNA and Cell Biology, Mary Ann Liebert, Inc., publishers, or their affiliates. No endorsement of any entity or technology is implied.