September 15, 2010 (Vol. 30, No. 16)
Richard A. A. Stein M.D., Ph.D.
Powerful and Versatile Tool Beginning to Demonstrate Significant Potential
One of the most challenging tasks in biomedical sciences is finding the experimental systems that are most informative about in vivo biological processes. In most instances, the road is long and arduous, and the implementation of better methodologies often takes time, even after their superiority is demonstrated and validated. Cell culture provides a fascinating example in this respect.
For decades, cells, often of a single type, have been cultured as monolayers growing in two dimensions in Petri dishes. While it is convenient and reproducible as a technique, in live organisms cells do not grow in two dimensions. Cells in live organisms are instead embedded, together with other cell types, in the extracellular matrix (ECM) to form 3-D structures. Two-dimensional cell culture appears, therefore, to be less than suitable as an in vitro tool seeking to accurately characterize the biology of tissues and organs.
“Three-dimensional cell culture is telling us much more about the physiology of normal organs and tumors than was available before,” said Mina J. Bissell, Ph.D., distinguished scientist at the Lawrence Berkeley National Laboratory.
Approximately three decades ago, Dr. Bissell was one of the first investigators to develop the concept of 3-D cell culture. In 1982, Dr. Bissell and collaborators introduced the concept of dynamic reciprocity, which proposed that the ECM, instead of just providing a structural anchor to the cells, signals through cell-surface receptors and the cytokeleton to the nucleus and chromatin, affecting gene expression. At the same time the nucleus signals back, establishing a highly dynamic and reciprocal interaction. This model was validated repeatedly.
Using 3-D cell cultures, investigators in the Bissell lab were able to dissect interactions between the microenvironment that surrounds mammary cells and cell behavior, showing that cells signal very differently in 3-D and 2-D.
One of the challenges of 3-D culture systems revolves not only around growing cancer cells but around how to grow non-neoplastic cells. Each type of cell possesses its own challenges. Cancer cells often produce their own ECM and the compounds they require for survival; they also form 3-D tumors when grown on agar. Non-neoplastic cells need the right substratum to form 3-D structures.
Non-Neoplastic Cells
“During work with non-neoplastic cells, one needs to provide what is necessary for them to form structures that will look and behave like a normal structure,” explained Sophie A. Lelièvre, Ph.D., associate professor at Purdue University.
The majority of cancers have epithelial origins, and to experimentally reproduce the phenotype of non-neoplastic epithelial cells, it is imperative to check for several things.
“One of them is polarity,” noted Dr. Lelièvre. All epithelial cells are polarized, with a basal side facing the ECM and an apical side facing the outside, which can be the blood vessel lumen, the lung alveoli, or the breast ductal system.
Dr. Lelièvre and collaborators have shown that maintaining apical polarity in epithelial cells is more sensitive to culture conditions than maintaining basal polarity. The full polarity of non-neoplastic cells is one of the most difficult aspects to preserve in vitro but represents one of the most important requirements to faithfully reproduce tissues phenotypically in vitro.
If cultures are grown with cells that are only partially differentiated, which means that they have basal but not apical polarity, those cells are already in a different state, they are already primed into the cell cycle, and the experimental conditions are not exploring a fully differentiated system.
“That is an important concept when studying cancer, because non-neoplastic tissue is fully polarized,” said Dr. Lelièvre. “In order to understand cancer, it is essential to first understand how a fully polarized cell becomes partially polarized. Otherwise, by starting with cells that are not fully polarized, one could miss an important step,” she added.
Dr. Lelièvre and collaborators have developed what they say is the first high-throughput culture system for non-neoplastic cells. This valuable tool will make it possible to study that impact of carcinogenic factors, such as environmental exposures, an important facet of preventive research.
New Systems
“We got into the area of 3-D cell culture because we needed to grow primary human kidney cells,” said John J. Gildea, Ph.D., assistant professor at the University of Virginia School of Medicine. As part of a larger consortium of labs, Dr. Gildea and collaborators are focusing on understanding the function of dopamine and angiotensin in hypertension.
“The main advancement that we implemented,” explained Dr. Gildea, “is that we placed magnetic beads inside a soft matrix, as opposed to cross-linked gelatin or plastic, and subsequently we were able to manipulate the magnet.”
Dr. Gildea and colleagues have used this technology, known as Global Eukaryotic Microcarrier (GEM™), for a couple of years. As compared to other technologies, GEMs do not have a rigid surface but are softer and more flexible. He reported that the placement of the matrix on top of a soft carbohydrate backbone provides a more accurate system to reproduce the in vivo organization of tissues, in which the viscoelastic structure created by hyaluronic acid fills the spaces between cells.
Other unique characteristics of GEMs are their low autofluorescence and the possibility to dissolve them and release the cells without proteases, which often interfere with downstream applications.
BioLevitator™, a benchtop incubator and bioreactor that uses GEM technology, was developed by Hamilton and Global Cell Solutions. The BioLevitator, which is reportedly the first benchtop 3-D cell culture system that allows GEMs to be kept in suspension with the ability to automatically culture them, minimizes the need for manual handling. The BioLevitator also provides stringent environmental control and allows four independent cultures to be processed simultaneously.
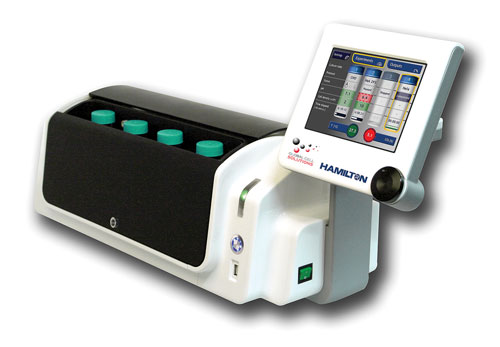
The BioLevitator is a benchtop incubator and bioreactor hybrid capable of handling four independent and high-density 3-D cell cultures. It eliminates peripheral instruments such as incubators and centrifuges and minimizes manual handling, according to Hamilton.
“The BioLevitator still allows manual media changes, which are the desired protocol in some laboratories. For labs with higher throughput looking for higher standardization, we also provide a fully integrated and automated solution, the 3D CellHOST. This system can integrate up to four BioLevitators and is based on the Hamilton Microlab Star workstation, our robotic platform. The 3D CellHOST fully automates parallel scale-up of different cultures,” noted Daniel Caminada, Ph.D., senior product manager in cell biology at Hamilton.
Three-dimensional cell culture promises to reshape many areas of investigation. “We came up with the idea to combine 3-D tissue engineering with drug discovery to improve the drug-screening process,” said Sanjit Nirmalanandhan, Ph.D., senior scientist at the University of Kansas Cancer Center.
Many drug candidates are identified with 2-D culture systems, but cells do not grow that way in live organisms. “That could be one of the reasons why most drug candidates identified with 2-D culture systems fail when they are examined in animal studies,” explained Dr. Nirmalanandhan.
When Dr. Nirmalanandhan and colleagues compared ten drugs, seven commercially available and three experimental, in 2-D cultures and in a 3-D lung cancer model that uses collagen as a scaffolding material, they found significant differences. While some drugs were more potent in the 3-D system, others were less potent, and a few did not show any differences.
This finding has significant implications for drug discovery. For example, a compound that is more potent in 2-D cultures, but less potent in the 3-D system, will not work well in an animal model. On the other hand, a compound that is potent in a 3-D system but not in a 2-D culture, might not be picked up during screening and would not even make it to animal testing, opening the possibility to miss valuable therapeutic agents.
“This finding highlights the problems associated with the current drug-screening system, which is not doing a good job in identifying successful candidates,” said Dr. Nirmalanandhan.
These considerations have huge practical implications for drug screening and promise better and more cost-effective methods but also open several challenges. “Without proper assays and imaging technologies, it will not be possible to conduct high-throughput assays in the 3-D format,” emphasized Dr. Nirmalanandhan.
An important concept in cancer biology is that tumors, as opposed to containing a single cell type buried in ECM, are made up of several cell types. Dr. Nirmalanandhan and colleagues are developing 3-D approaches to co-culture lung cancer cells with other cell types to create blood vessels, which would provide an excellent model to screen drugs that either kill the neoplastic cells or perturb vascularization, which are the two major ways to treat tumors.
“The field has turned the corner,” said Matthew R. Gevaert, Ph.D., CEO of Kiyatec, a company that he co-founded. “3-D is better and much more physiologically relevant than 2-D, this is intuitive, and there is a lot of data to back it up.”
Kiyatec recently released Kiyakube™ 3D Cell Culture Plasticware, which Dr. Gevaert said was the first product in a technology platform that allows standardized 3-D cell culture. It can accommodate any type of scaffold material, he reported, allowing users to conduct in situ confocal microscopy and convenient sampling.
“I believe the next scientific and market demand will be segregated co-culture, allowing users to set up 3-D cultures with several tissue types having controlled exchange of soluble factors,” he predicted.
At CHI’s “Bioprocessing Summit” held last month, Rebecca Drumm, cell culture scientist at Kiyatec, talked about research that compared exponential growth of HepG2 cultures in 2-D with stable HepG2 populations in cells grown under 3-D conditions, suggesting a more regulated cell proliferation rate that is comparable to the situation in vivo. In addition, cells grown in dynamic 3-D culture produced more albumin than those grown in static 3-D culture, showing functional properties closer to in vivo conditions.
GEM™ magnetic microcarriers were designed to maintain the in vivo phenotype of cell lines while providing a vehicle for culture transport through the drug discovery process. GEM not only supports high-density cell culture but also can be controlled throughout medium exchange or culture collection during manual or automated handling, according to Global Cell Solutions.
Building on NASA Research
“We are taking 3-D cell cultures to the next step,” said Bill Anderson, president and CEO of Synthecon.
Approximately two decades ago, the Johnson Space Center at NASA developed a new type of cell culture system based on the principle of clinorotation, in which slow rotation of a fluid-filled culture vessel leads to the nullification of the gravitational forces. This low-shear, low-turbulence growth environment allows cells to adopt phenotypes that are normally not observed during growth in 2-D cultures. One of these phenotypes is the creation of 3-D structures that resemble in vivo tissues.
Synthecon further designed and patented several innovations to this application and is currently marketing a rotating wall vessel bioreactor, known as Rotary Cell Culture System (RCCS), which allows users to grow dynamic cultures in which the matrix, suspended in a fluid bath, promotes 3-D cell growth.
By using the rotating wall vessel bioreactor, investigators have shown that Huh7 hepatoma cells grown in 3-D rotating wall vessels are morphologically and functionally different than the same cells grown in 2-D cultures. These cells became permissive for hepatitis C virus infection, which allowed investigation of the infection process.
Additionally, RCCS technology enables several cell types to be co-cultured to generate relevant 3-D tissues. “The ability to co-culture multiple cell types to form physiologically relevant tissues really sets Synthecon apart from the rest of the 3-D technologies in the field that only support 3-D monoculture on matrix,” emphasized Anderson.
While 3-D cell culture promises to re-shape the way we approach many questions in life sciences, it is still a technology under continuous development and improvement.
“Three-dimensional is not the end of the challenge,” explained Dr. Bissell. “Everything is context-dependent and tissue- and organ-specific. We need 3-D models for every organ, and these will be different in terms of their requirements and their complexity. We need to understand the physiology and the biology of each organ we are working on, and we cannot be answering universal questions with one model system.”
The need to use the most relevant and most informative in vitro models for each individual scientific question is a priority in all fields of investigation. Three-dimensional cell culture will catalyze significant changes in many areas and will pave the tortuous but rewarding road of scientific discovery, which still harbors so much unknown. As Lewis Thomas eloquently wrote a few decades ago, “Biological science…is under way, but only just under way.”
Sidebar: 3-D Scaffolding Company Moves Forward Quickly
Founded just three years ago a New Brunswick, NJ-based high-tech firm is already making its mark in the area of 3-D cell culture devices for stem cell/tissue engineering and drug discovery applications. 3D Biotek bases its approach on precision 3-D microfabrication technology and advanced biomanufacturing techniques, according to company officials.
The National Institute of Standards and Technology recently reported that it will use 3D Biotek’s polycaprolactone scaffolds (3D Insert™–PCL) as Reference 3-D Tissue Scaffolds. This came right after Marika Bergenstock, Ph.D., a post-doc research associate working in a 3D Biotek lab, received a STAR award from the Society for Biomaterials for her paper—“Engineered Polystyrene Scaffolds For In Vitro Three-Dimensional Disease Models.”
Last year, 3D Biotek won the NJ Technology Council Award as the “2009 Incubator Company to Watch” and a $195,000 Edison Innovation R&D grant from the NJ Commission on Science and Technology to develop bioactive bone and cartilage repairing scaffolds.
Qing Liu, Ph.D., company founder and CEO, believes the secret to the firm’s rapid growth is the result of a combination of hard work and a little bit of luck.
“Each employee is dedicated to making the company a great success,” he said. “Also, 3D Biotek’s management team chose the right direction to move forward, creating successful partnerships and collaborations and bringing novel and indispensable products to the market.”