April 1, 2011 (Vol. 31, No. 7)
Carol Potera
Mymetics Seeks to Prevent Infection with Vaccines that Induce IgA Antibodies at the Mucosal Level
After more than 25 years of research, a vaccine to prevent AIDS remains elusive. A new vaccine being developed at Mymetics is drawing attention because of its unique approach. The vaccine provides both first-line mucosal and second-line cellular immunity, according to the company. It is designed to block early transmission of the virus at mucosal surfaces, preventing it from spreading throughout the body.
Called MYMV101, the vaccine combines a peptide that stimulates mucosal immunity with a modified gp41 antigen for additional cellular immunity. The antigens are delivered via virosomes, which are lipid membranes modeled after envelope viruses. These lipid-based carriers contain functional fusion viral proteins and rationally designed antigens. Virosomes fuse with target cells, but they do not replicate. The virosome platform was created at Berna Biotech and improved by Virosome Biologics, which Mymetics acquired in 2009.
The route of administration of MYMV101 is unusual, too. The vaccine is first injected intramuscularly then followed by an intranasal booster dose. This approach induces IgA antibodies at mucosal surfaces to block viral entry, as well as IgG antibodies circulating in the blood. “Our vaccine represents a first line of defense before the virus can settle in the tissues and spread within the body. Targeting a virus before it enters the body is probably more efficacious than after it invades the body and infects it,” says Jacques-Francois Martin, CEO.
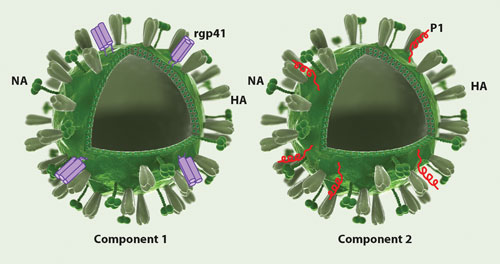
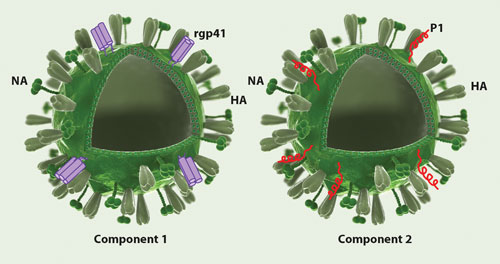
Mymetics’ HIV-1 preventive vaccine is based on gp41-derived antigens attached on the surface of virosomes that act as an antigen delivery system. Virosomes are nonreplicative vectors that are essentially spherical lipidic vesicles, representing reconstituted empty influenza virus envelopes harboring the hemagglutinine (HA) and neuraminidase (NA) viral proteins, devoid of the genetic material. The lipidated antigens (recombinant gp41 proteins on the left and P1 peptides on the right) are intercalated into the lipid membrane of virosomes through the lipid tail.
Clues to IgA Protection
Mymetics researchers were inspired by observations that some men and women in Africa remain HIV-negative, despite being regularly exposed to HIV during unprotected sex. Morgane Bomsel, Ph.D., at INSERM in Paris, a key collaborator with Mymetics, discovered the reason for this natural resistance—the vaginal and rectal secretions of resistant individuals contain specific IgA antibodies. Dr. Bomsel showed that HIV enters human mucosal tissue by a transcytosis mechanism, and that IgA antibodies against a specific part of the gp41 protein could block this entry.
Most pathogens enter their hosts through mucosal surfaces, such as the respiratory, genito-urinary, or gastrointestinal tracts. They migrate in the bloodstream to organs where they replicate. Classic vaccines trigger mostly IgG antibodies in blood, but not IgA antibodies at the point of entry. Based on Dr. Bomsel’s results, researchers at Mymetics designed a vaccine formulation that induces IgA antibodies at the mucosal level. Most other HIV immunization projects have targeted IgG antibodies, or specific cytotoxic cells against HIV in the blood.
Strong Proof-of-Concept Evidence
Blocking mucosal entry is proving highly promising, yet it is still a poorly investigated route. Mymetics scientists presented preclinical results obtained from research on macaques that were either immunized with MYMV101 or left nonimmunized. After repeated challenges with HIV, all the animals that were immunized both intramuscularly and intranasally remained uninfected, whereas 100% of the nonimmunized controls became infected.
In contrast, when macaques were immunized only intramuscularly and not given the intranasal booster, just half of them were protected from HIV challenges. “Injections alone raise lower levels of immunity, and the intranasal application is needed to optimize mucosal immunity,” Martin explains.
Next, a Phase I trial involving 24 women showed that the vaccine induced not only serum antibodies but also mucosal antibodies in the genital and intestinal tracts in most of the volunteers. In the past 25 years, few other HIV vaccine candidates have elicited both blood and mucosal antibodies, Martin notes.
Conducted at the Center for Vaccinology at the University of Ghent, Belgium, the women received two injections of the vaccine, followed by two doses given as a nasal spray. The preliminary results in macaques and humans “are an important validation of our work and approach.”
In addition to HIV/AIDS, Mymetics has four other vaccines in the pipeline: influenza, respiratory syncytial virus (RSV), malaria, and herpes simplex virus (HSV). All are based on the virosome delivery technology, and with the exception of the malaria vaccine, stimulate mucosal immunity.
Solvay Pharmaceuticals (now Abbott Pharmaceuticals) licensed the influenza vaccine, a totally intranasal application aimed at the elderly, and is starting Phase II testing. The RSV and HSV vaccines are in preclinical testing. The malaria vaccine completed a Phase Ib trial in Tanzania where it was well tolerated and triggered long-lasting antibody responses. Mymetics plans to develop its vaccines through Phase II trials, then license them to pharmaceutical companies.
The vaccine world has viewed Mymetics’ mucosal approach with skepticism. However, “when the macaque results were made public, it became difficult to ignore that our approach is valid,” says Ronald Kempers, CFO. The Gates Foundation, National Institutes of Health and other international agencies are watching the progress of the Mymetics platform.
The lack of popularity of mucosal vaccines stems partly from a failed influenza vaccine given intranasally that was marketed by Berna. Because of serious side effects, such as facial pain, it was withdrawn from the market. “This discouraged others from making nasal mucosal vaccines,” says Martin.
Since then, scientists at Mymetics learned that the adverse effects of the Berna vaccine were due to an adjuvant, not the vaccine itself. “We do not need adjuvants with our virosomes, and we hope this makes people more comfortable with the idea of mucosal vaccines.”
It was also assumed that a mucosal vaccine had to be given at the primary site of infection, such as the respiratory tract to prevent influenza. The results of the Phase I trial proved otherwise. “When given as an intranasal application, mucosal antibodies were induced vaginally and rectally,” says Martin. He hopes that the results will attract new funding opportunities.