September 1, 2009 (Vol. 29, No. 15)
K. John John Morrow Jr. Ph.D. President Newport Biotech
Automation, Improvements in Gel Elution, and Use of Nanostructures Advance Methodologies
Mass spec has emerged as a powerful and versatile technology for the exquisite characterization of molecules. Fast, sensitive, and economical, it provides a wealth of information that previously could only be obtained through lengthy and complex protocols.
As an increasingly user-friendly technology, it has reached a stage where it is now ubiquitous throughout the biotech industry. A major component in protocols for processing biological materials for mass spec is sample preparation, so it is not surprising that a section of the recent ASMS conference was given over to this topic.
“A major concern in the development of bioanalytical methods is the effect of the biological matrix on detection,” said Craig Aurand, senior application chemist, analytical research services at Supelco, a division of Sigma-Aldrich. He described his team’s experiences with interference in quantitation due to coextraction of the matrices, which results in introduction of irregularities into the subsequent chromatographic analysis.
According to Aurand, robotic automation combined with the use of narrow high-efficiency columns has greatly expedited analysis but increased the risk of matrix effects.
In a quest to improve protein precipitation techniques, Aurand and his colleagues evaluated the standard approach (reverse-phase gradient elution chromatography using a two-step sample-prep procedure) to determine the impact of extracted biological matrix on analyte response. Plasma samples were prepared according to conventional protocols. The standard approach was then compared with samples that were processed using specially configured phospholipid depletion 96-well plates combined with hydrophilic interaction chromatography aqueous normal-phase (HILIC/ANP) chromatographic conditions.
According to Aurand, the new technique is a simplified approach that results in high analyte recovery while at the same time removing the phospholipid matrix from the plasma samples. Increased analyte response with shorter run times is another benefit.
Using this new platform, Aurand reported that his group was able to reduce analysis time from the 20 minutes typically required to elute phospholipids from standard protein precipitation samples to 90 seconds. “We’re moving forward with our platform, evaluating different iterations, and miniaturizing the sample size so that we can get by with smaller and smaller volumes of plasma,” he said.
Human Plasma Renin Activity
Kheng Lim, Ph.D., principal scientist, and Daniel B. Kassel, Ph.D., vp of analytical sciences and DMPK at Takeda Pharmaceuticals, investigated renin inhibitors, a group of compounds employed for the treatment of hypertension, chronic renal disease, and congestive heart failure. Human plasma renin activity is reflective of the performance of the renin-angiotensin system and serves as a basis for the diagnosis of hypertension.
The renin-angiotensin system regulates blood pressure and fluid balance through a feedback mechanism in which the kidneys respond to low blood volume by secreting renin. This causes angiotensin levels to rise and blood vessels to constrict. So-called ACE inhibitors are important pharmacological agents used to block one of the critical steps in angiotensin II production. Typically, a radioimmunoassay (RIA) has been used to determine plasma levels of renin during the identification and investigation of inhibitory compounds.
In vitro human plasma renin activity assays incorporating radiolabeled angiotensinogen have been used routinely within the industry for identifying renin inhibitors. Compounds are spiked into human plasma, and the generation of angiotensin I from the enzymatic cleavage of angiotensinogen by renin is measured. “One of the major drawbacks to the RIA is that its linear dynamic range is not large,” Dr. Lim noted. “The standard curves level off rapidly, thus limiting the performance.”
There have been efforts to develop LC/MS/MS as a means for quantifying endogenous levels of angiotensin I; however the assays were time consuming and tedious, hardly representing an advance over the radioimmunoassay approach. Drs. Lim and Kassel have been working on an improved method that uses online solid-phase extraction. “Our online sampling approach replaces manual sampling and can save up to a full-time equivalent position,” Dr. Lim said.
Their system consists of an Applied Biosystems API 4000™ triple quadrupole mass spectrometer (Life Technologies), Shimadzu binary pumps, and a CTC PAL autosampler. In a series of experiments, Drs. Lim and Kassel optimized the procedure, evaluating different types of solid-phase extraction cartridges, analytical columns, and finally, the injection, wash, and elution steps.
“We found that the online SPE/LC/ MS/MS option for the human plasma renin assay is practical and robust,” Dr. Lim stated. “Indeed, we believe that the application of online solid-phase extraction could be extended to the analyses of small molecules, endogenous peptides, and other plasma biomarkers.”
Peptide Peak Areas
Joomi Ahn, senior research chemist in the biopharmaceutical sciences department at Waters, and John R. Engen, Ph.D., associate professor at Northeastern University, have been pursuing further improvements in hydrogen/deuterium exchange mass spectrometry (HXMS), specifically the degree of reproducibility of the pepsin digestion data.
HXMS involves a chemical reaction in which a covalently bonded hydrogen atom is replaced by a deuterium atom or vice versa. Usually the examined protons are the amides in the backbone of a protein, and because hydrogen exchange gives information about the solvent accessibility of various parts of the molecule, it is an important approach for generating descriptions of protein tertiary structure.
A critical feature of the procedure is the production of peptides through online acid protease digestion. By using a nonspecific protease immobilized on a column, the samples can be moved through the process train without additional isolation or purification steps.
The researchers digested a variety of proteins with pepsin and then measured the peak areas in a large-scale replicate analysis using LC/MSE methodology. The studies were performed using Waters’ nanoAcquity UPLC® and Synapt™ HDMS™ systems. The listed peptides, consistently identified more than 27 times out of 31 runs, showed an average of 6.6% relative standard deviation of the peak area, indicating a high level of reproducibility.
Peptic peptide maps resulted in extensive sequence coverage for the majority of the proteins studied, up to 100%, including in some cases the mapping of post-translational modifications and disulfide bonds by the chromatographic separation in less than 10 minutes.
“The high confidence and accuracy of peptide identification demonstrates the low variability and good reproducibility of the UPLC separation technology for analysis of these complex pepsin digestion samples,” Ahn said. “This information is highly significant for reliable conformational studies of proteins. Moreover, the rapidity of the analysis has piqued the attention of investigators using the widely applied trypsin digestion procedure,” whose adaptation for online analysis, she said, could be onerous.
Grappling with Sample Complexity
One of the biggest challenges in the field of proteomics is dealing with the enormous complexity found in biological samples. In a typical GeLC workflow, protein bands separated by 1-D polyacrylamide electrophoresis must first be sliced by hand and digested to liberate associated peptides into solution before analysis via mass spectrometry can be realized. In addition to being tedious, this process provides limited yield and restricts downstream analysis to tryptic peptides, rather than intact proteins.
To aid researchers in this process, Protein Discovery introduced the GELFREE™ 8100 Fractionation System. The GELFREE (Gel Elution Liquid FRaction Entrapment Electrophoresis) technology allows molecular weight fractionation using high-resolution PAGE with recovery of intact proteins in the liquid phase.
As analytes in the sample are separated, the molecular weight fractions elute from the end of the gel and are concentrated in a liquid entrapment zone. The eluted fractions are then removed with a standard pipette and are ready for downstream preparation and analysis using conventional mass spec instrumentation.
The GELFREE design features an independently controllable, eight-channel electrophoretic instrument capable of simultaneously supplying constant current or voltage to each of the eight channels in the cartridge with pre-set fraction collection steps defined through the onboard touch screen interface. All eight channels can be run in parallel and yield 15–20 fractions per channel in less than 90 minutes, according to Chuck Witkowski, president and CEO.
“The system provides highly reproducible fractionation based on molecular weight with high yield recovery in the liquid phase. Since proteins are recovered intact, valuable information such as post-translational modifications and truncations can be readily determined,” stated Witkowski.
The fractions are nonbiased, added Witkowski, who noted that recovery from GELFREE is greater than 60% across the mass range. Cartridges are provided with precision precast gels, enabling reproducibility. Up to 1 mg of total protein can be loaded on each channel, so low abundance proteins can be visualized.
LC-NALDI
Liquid chromatography combined with nanostructure-assisted laser desorption ionization (LC-NALDI) was the topic of a presentation by Sergei Dikler, Ph.D., senior application scientist at Bruker Daltonics. NALDI refers to targets that have active surfaces consisting of layers of nanostructured inorganic materials with a hydrophobic organic layer on top. These surfaces are optimized for matrix-free sample deposition from various mixtures designed for high-throughput analysis. “In our studies we have demonstrated the value of nanostructured targets for analyzing peptide fractions in a matrix-free LC-LDI proteomics workflow.”
LC-LDI is a matrix-free variation of an LC-based proteomics workflow in which NALDI targets spotted during an LC run are analyzed offline on a MALDI-TOF/TOF mass spectrometer.
“In order to demonstrate the utility of this approach, we analyzed commercially obtained tryptic digests with TOF/TOF mass spectrometry followed by visualization of the results using WARP-LC SurveyViewer and ProteinBrowser,” Dr. Dikler explained.
Sorting Out Ubiquitination
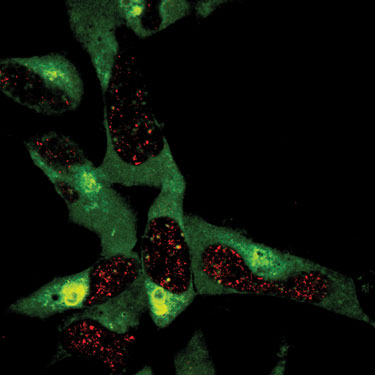
“We are following the process of polyubiquitination through the use of mass spec,” stated Eric Dammer, Ph.D., a postdoctoral fellow in the lab of Junmin Peng, Ph.D., assistant professor in the department of human genetics at Emory University School of Medicine, “with the long-range goal of developing new drugs for treating mental disorders.”
In order to investigate the ubiquitin trafficking process, Dr. Dammer and his colleagues used mass spec combined with SILAC (stable isotope labeling with amino acids in cell culture). This is an ideal technology for establishing linkages of polyubiquitin to target proteins, but it requires careful optimization of the LC/MS/MS parameters, including loading amount and gradient length.
By systematically adjusting these properties, they were able to identify proteins and investigate relationships between loading amounts and gradient lengths that can maximize protein identification. Knowing the appropriate settings and parameters is critical in mass spectrometry as frequently sample amounts are limited and precious.
The group did these investigations using the Thermo Scientific LTQ Orbitrap XL ETD (Thermo Fisher Scientific), which was specifically designed for proteomics analyses. It combines three different and complementary fragmentation techniques, collision-induced dissociation, higher-energy collisional dissociation, and electron transfer dissociation.
Sample preparation for mass spec analysis is a critical component of the technology, since the full potential of the hardware cannot be realized without high-quality preps. Moreover, in today’s laboratories high throughput is also essential in order to match the rapid analysis of which the instruments are capable. For this reason these developments will be followed with great interest in the coming years by those in the field.
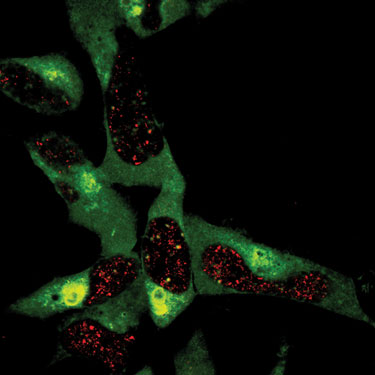
Scientists at Emory University School of Medicine are investigating the polyubiquitination process using the Thermo Scientific LTQ Orbitrap XL ETD.
K. John Morrow Jr., Ph.D. ([email protected]), is president of Newport Biotech and a contributing editor for GEN. Web: www.newportbiotech.com.