March 15, 2011 (Vol. 31, No. 6)
Vicki Glaser Writer GEN
Recent High-Profile Success of Dendreon’s Provenge Has Not Quelled Widespread Skepticism and Skittishness
At Phacilitate’s recent “Cell & Gene Therapy Forum”, enthusiasm greeted reported advances in the research laboratory and successes in the commercial arena for technologies that represent high-risk ventures for the companies developing them. Excitement also accompanied the introduction of pioneering therapeutic strategies that epitomize the promise of personalized medicine.
Sharing the spotlight with the research and clinical news, however, was an omnipresent reminder of scaleup and manufacturing challenges, regulatory and reimbursement hurdles, cautious acceptance by the healthcare community, and skepticism and skittishness among investors—particularly in the current economic climate.
The push-and-pull between optimism for the future and the reality of overcoming current challenges was evident—one presentation was entitled, “So you’ve got a C> product that works—now, how are you going to make money from it?”
The focus of many of the talks was Dendreon’s autologous cellular immunotherapy, sipuleucel-T (Provenge®), which received FDA approval nearly a year ago for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer. In January, Dendreon reported $48 million in 2010 revenues for Provenge and announced plans to seek marketing authorization in Europe. The company plans to increase production capacity by about 10-fold in 2011, with concomitant increases in its sales force and its marketing and physician/patient education efforts.
A key topic of discussion was how cell and gene therapies will be assessed by insurers for reimbursement. Robyn Karnauskas, Ph.D., director and research analyst at Deutsche Bank Securities, gave a sobering appraisal of the current investment picture as she summarized the lessons learned from the Dendreon experience. Increasingly cautious investors want to see positive healthcare outcomes to assess the potential of a company’s new drug or therapeutic intervention.
Demonstrating a promising response rate is no longer enough; companies need to show survival data and “superclean trial results,” said Dr. Karnauskas. This will entail longer, more expensive trials and increased risk. She described an underlying fear among investors of drugs with novel mechanisms as well as growing concern around biosimilars.
The news from the investment arena is not all disheartening, though, as Dr. Karnauskas noted a rising trend in IPOs and the availability of funding for companies that prove worthy.
She emphasized the uncertainty surrounding regulatory and reimbursement factors as well. The FDA “has set the bar higher for approval” since about 2009, she said, and companies need to demonstrate a clear clinical strategy for development. She described the recent FDA decision to withdraw its approval of Avastin in breast cancer due to the drug’s side effects as “not expected.”
Additionally, “reimbursement has become more challenging,” said Dr. Karnauskas, as evidenced by Medicare’s restrictions on Epogen to treat anemia associated with dialysis and the Alzheimer disease drug Aricept. The Centers for Medicare and Medicaid Services has initiated a National Coverage Analysis of Provenge to determine reimbursement.

Dendreon says its Provenge was the first autologous cellular immunotherapy to receive FDA approval for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer.
Ramping Up
Clifford Goodman, Ph.D., vp at The Lewin Group and chair of the Medicare Evidence Development & Coverage Advisory Committee, emphasized the importance of comparative effectiveness research (CER) and its role in advancing personalized medicine. CER requires a direct comparison of alternative interventions—head-to-head comparisons of new treatments against standards of care, not placebo—and reporting of health outcomes. It is not sufficient to rely on response rates or increased/decreased levels of surrogate biomarkers as indicators of efficacy.
“Personalized medicines must show that an intervention has some direct, or at least demonstrably indirect, favorable impact on health outcomes in real-world practice settings,” said Dr. Goodman.
Furthermore, “for CER to contribute to personalized medicine, it will have to emphasize priorities and study designs that account for individuals’ genetic, behavioral, environmental, and other personal traits,” he added. “CER needs to incorporate subgroup analyses,” as mean effects derived from an entire trial population are not necessarily indicative of a treatment’s effect in an individual or subpopulation (a concept known as heterogeneity of treatment effects).
Representatives of Dendreon shared the company’s experiences and strategies in the development and ongoing commercial roll-out of Provenge. The therapy is derived from peripheral blood mononuclear cells obtained by leukapheresis and activated with a combination of recombinant prostatic acid phosphatase and granulocyte-macrophage colony-stimulating factor to yield CD54+ T cells that are expanded ex vivo and returned to the patient.
Michael Covington, Dendreon’s CMC director, regulatory affairs, spoke about the need to demonstrate that a product made at multiple manufacturing sites is the same. To limit the need for repeated batch-to-batch testing, Covington suggested instead that product comparability can be demonstrated by documenting comparability of the GMP systems in place at different facilities and by thorough product characterization.
Robert Preti, Ph.D., is president and CSO at Progenitor Cell Therapy, a CMO that produced Provenge for use in Dendreon’s Phase II and III trials. He stressed the value of having a well-characterized product and of building in desirable traits, such as longer shelf-life, earlier in development. He said there’s no need to recreate the wheel when facing the challenges of commercial development of autologous cell therapies. There is much to be learned from the blood industry and from the experiences of antibody and biopharmaceutical manufacturers, he noted.
David Smith, head of global therapeutic cell solutions at Lonza, described the company’s work on allogeneic cell therapy beginning in 2008 and the strategies devised to reduce the cost of goods and bring down the initial $500 per dose cost of an allogeneic cell therapy. By optimizing scaleup, facility design and utilization, and downstream processing, Lonza is now able to manufacture the product for less than $250/dose.
Lonza is now applying a similar strategy to the production of autologous cell therapy products. The company recently constructed cGMP high-throughput manufacturing facilities in Walkersville, MD, and in Singapore. As manufacturing processes are scaled to commercial levels, the main cost driver is product testing, Smith explained. To help bring down manufacturing costs, companies need better informatics, more automated and high-throughput testing methods, and improved regulatory strategies, cost-of-goods logistics, and extended product shelf-life.
Scaling Out
For autologous cell therapies, moving to large-scale manufacturing means scaling out rather than scaling up. That is, being able to repeat the same processes on a larger number of samples rather than producing a larger quantity of any one product.
What are the biggest issues related to scale-out? The speakers highlighted three: shifting from manual to automated processes; maintaining chain of custody (ensuring that the cells that came out of a patient are the same cells returned to that patient); and scalability of testing (minimizing the amount of product lost to testing and the time delay in product delivery).
Speakers from Agenus (formerly Antigenics), which is developing the Prophage series of autologous cancer vaccines, and Opexa Therapeutics, developers of autologous T-cell therapies, presented operational models for global delivery of autologous cell therapies and personalized vaccines that rely on one or a few centralized manufacturing facilities and decentralized, regional Centers of Excellence for collection of cell samples and delivery of the product to patients.
The aim of decentralizing cell collection and product delivery is to make the treatments more accessible to patients. At the same time companies need to ensure a rigorous chain of custody and to minimize the time from cell harvesting to delivery of a cell therapy.
Agenus’ Prophage vaccines (marketed as Oncophage® in Russia to treat renal cell carcinoma) are in Phase II trials in the U.S. to treat glioma (a type of brain tumor) and pediatric neurological tumors. Opexa’s Tovaxin® is comprised of patient-specific attenuated myelin reactive T cells intended to stimulate an immune response against pathogenic demyelinating T cells in patients with multiple sclerosis. The therapy has completed a Phase IIb study in patients with relapsing/remitting MS.
Highlighting Advances in Automation and Stem Cell Culture
At the meeting, Thermo Scientific featured its Nunclon™ Vita™ energy-treated polystyrene surface for the growth and expansion of human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) without the need for feeder cells or extracellular matrix.
The company uses energetic oxidation to induce a chemical reaction in the polystyrene polymer that creates functional groups onto which hESCs can attach and form colonies. A patent-pending method that combines the Nunclon Vita surface and conditioned media containing the Rho-associated kinase inhibitor Y27632 allows for the growth of hESCs for >10 passages and of iPSCs for >3 passages without differentiation or karyotype changes, according to Thermo.
U.K.-based Angel Biotechnology, which specializes in GMP manufacturing of cell therapies including stem cells, viruses, antibodies, and recombinant proteins, recently announced a new GMP manufacturing contract and a five-year pricing agreement with Russian pharmaceutical company Materia Medica. Angel plans to increase its manufacturing capacity by at least fivefold.
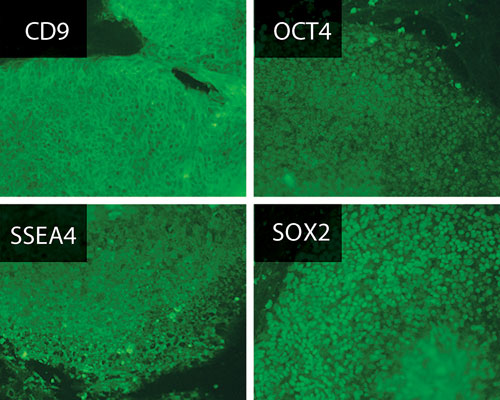
Thermo Scientific’s Nunclon Vita surface was designed for the growth and expansion of hESCs or IPSCs. It does not require feeder cells or ECM. Expression of pluripotency markers in human ESCs as determined by immunofluorescence staining after 11 passages on Nunclon Vita surface.
Automated Processing
CaridianBCT launched the Quantum Cell Expansion System, an automated, closed system that supports the growth of adherent and suspension cells in a hollow fiber bioreactor. Cell loading, media exchange, and cell harvesting are all automated. The seven-minute harvesting step can reportedly yield 0.5–1 billion cells.
Later this year, Miltenyi Biotec will introduce the CliniMACS® Prodigy integrated cell-processing system. The customized system can process cells from blood or bone marrow, carrying out all operations—from cell washing and density gradient separation to MACS® cell separation and cell harvesting—in a closed environment.
The Automation Partnership (TAP) designed the CellBase CT cell-expansion system to meet the needs of companies transitioning autologous cell-therapy products from manual to automated processing under GMP conditions to produce quantities needed for clinical and commercial development. The system can maintain up to 90 T flasks under temperature and CO2 control, and provides noncontact media dispensing and flask-to-flask transfer, automated cell count and cell viability analysis, confluence measurements, and barcoding for sample tracking.

CaridianBCT’s Quantum Cell Expansion System is an automated system designed for growing adherent and suspension cells in a closed environment. Based on hollow-fiber bioreactor technology, it allows for large-scale manufacturing of cells under GMP control with a reduced risk of contamination, stable incubation, and continuous cell feeding and waste removal using bags organized above the device.
Leading the Pack
ReGenX highlighted its recombinant adeno-associated virus (AAV) vector technology for tissue-targeted in vivo gene delivery. The technology can be used to overexpress a gene or inhibit gene expression or activity. Applications include creating animal models of disease and screening therapeutic proteins in vivo. Specific AAV serotypes allow for gene transfer to targeted tissues including heart, retina, brain, lung, pancreas, liver, and skeletal muscle.
Eufets provides product-development services for cell and gene therapies including process development and scale-up, cGMP contract manufacturing, assay development, quality control, preclinical studies, and regulatory support.
Advaxis is pursuing multiple Phase II trials in cervical cancer and head and neck cancer for its live-attenuated Listeria monocytogenes vaccines targeting human papilloma virus. The company also has Listeria monocytogenes therapeutic vaccines in clinical studies in breast and prostate cancer and in glioma. The vaccines contain the Listeria monocytogenes virulence factor listeriolysin O, a protein that stimulates an innate immune response and a cytokine response that enhances the function of antigen-presenting cells.







