August 1, 2009 (Vol. 29, No. 14)
Mats Boren, Ph.D.
Stabilizor System Aims to Preserve Proteins in a Biologically Relevant State
The importance of understanding the role of proteins in diseases—as drugs, drug targets, and biomarkers—has increased remarkably in recent years. In fact, it is now undeniably clear that proteins play an important role in the discovery of remedies for a wide range of serious diseases. A more detailed picture of the proteome is now available; and the serious risks of inducing protein changes post-sampling and throughout analysis are also now apparent.
Intrinsic enzymatic activity results in protein degradation and loss of post-translational modifications. As a direct consequence, analytical results reflect a mixture of the in vivo proteome and its ex vivo degradation products, leading to confounding results that don’t reflect the true biological situation and immensely increase the risk of false biomarker discoveries. The issue of post-sampling changes must be adequately addressed in order to realize the full potential of protein studies.
Sample Preparation
Throughout the sample-preparation process, freeze/thaw steps and homogenization facilitate enzymatic activity by providing access to substrates not normally encountered by enzymes, thereby accelerating degradation. Denator has developed a system that eliminates all enzymatic activity (Figure 1).
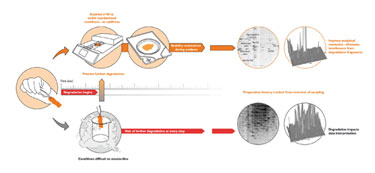
Figure 1. Use of the Stabilizor leads to denaturation and permanent inactivation of enzymes.
Use of the Stabilizor™ T1 (Figure 2), which is based on rapid and uniform thermal inactivation, leads to denaturation and permanent inactivation of enzymes. This is accomplished by exposing the sample to conductive heating, assisted by vacuum suction and pressure during a specific period of time—this ensures efficient and complete stabilization of the sample.
Stabilizor T1 measures the sample with a laser and automatically assesses the optimal treatment for each sample, guaranteeing reproducibility. Frozen samples can be stabilized as is, thus preventing the massive proteolytic degradation that occurs during thawing. Stabilized samples can be analyzed using all major protein study workflows and standard protocols with only minor adjustments.
The addition of chemical enzyme inhibitor cocktails or manipulations such as cross-linking or pH alterations are common means of minimizing post-sampling degradation. Rapid and homogeneous thermal inactivation has advantages over other methods that are either reversible, added during later stages, not suitable for specific downstream analysis techniques, or impractical or limited in some other way, e.g., toxic or expensive.
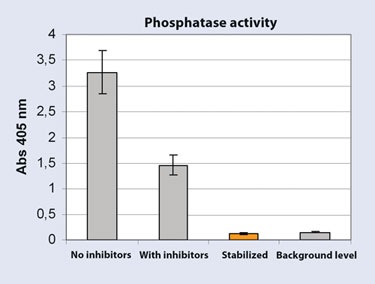
To demonstrate the efficiency of the Stabilizor system, phosphatase activity was compared in protein extracts from stabilized and nonstabilized mouse brains, extracted with and without phosphatase- and protease inhibitors. The results show high activity in extracts from nonstabilized brain. Roughly half of the activity remained despite the presence of inhibitors during extraction. In stabilized samples, activity levels were detected at background levels, thereby showing that enzymatic activity is eliminated following Stabilizor treatment and that there is no further risk of enzymatic degradation or changes occurring in stabilized samples (Figure 3).
In addition, Western blot analysis of phosphorylated CREB, GSK3b, and ERK1/2, show that stabilized samples conserve phosphorylations for up to two hours at room temperature. When the same proteins were analyzed after only 10 minutes post-mortem in snap frozen samples, they were detected at significantly lower levels, indicating extensive ex vivo dephosphorylation.
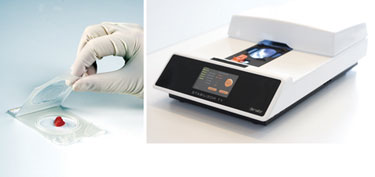
Figure 2. Stabilizor T1 instrument and Maintainor Tissue consumable
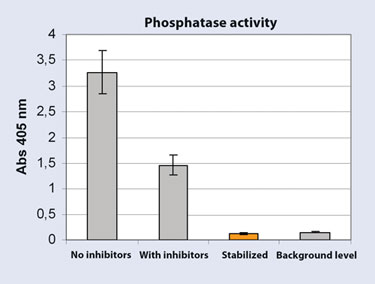
Figure 3. Phosphatase activity measured using SensoLyte pNPP phosphatise kit (Anaspec)
Case Studies
Two-dimensional gel electrophoresis (2-D GE) is a proven tool in the protein study toolbox. To minimize protein degradation, inhibitors are routinely added to the urea-based buffers used for protein extraction. The effect of the Stabilizor system was tested on this relatively degradation-safe system in Professor Michael J. Dunn’s laboratory at the Proteome Research Centre, UCD Conway Institute, Dublin, Ireland.
Mouse brains were harvested and either directly frozen, stabilized before freezing, or stabilized from a frozen state. Large differences were detected between stabilized and nonstabilized samples with a clear trend of fewer low molecular weight (LMW) spots and higher intensities of high molecular weight (HMW) spots in stabilized samples compared to nonstabilized.
Decreased LMW spots were most often subsequently identified as degradation fragments, whereas increased HMW spots were mostly identified as full-length proteins. This indicates that despite the use of state-of-the-art inhibitors and urea buffers, degradation still remains a problem that may lead to misleading results.
A second case study examined phospho-specific immunohistochemistry with the department of pathology and cytology at Uppsala University in Sweden. Protein phosphorylations modulate activity and sub-cellular localization and are frequently involved in cell signaling. Phosphorylation states change rapidly in response to external stimuli and are often drastically affected by post-mortem changes. Formalin, which is routinely used to fixate tissue samples prior to IHC, penetrates tissue slowly, ~0.5–2 mm/h, allowing for extensive changes in phosphorylation states prior to complete fixation.
The stabilization of phosphorylations with the Stabilizor system was evaluated using phosphospecific antibodies, here exemplified with CREB phosphorylated on Ser133 (pCREB(Ser133)). Mouse brains were either directly fixed in formalin or stabilized prior to formalin fixation.
To assess stability of pCREB(Ser133) at room temperature, samples were incubated at room temperature prior to, or after, stabilization. In samples directly subjected to formalin fixation, staining was primarily localized close to the tissue surface, consistent with loss of phosphorylations during formalin penetration.
In stabilized samples, pCREB positive staining could be seen throughout the tissue. In nonstabilized tissue, all traces of pCREB(Ser133) staining were gone after only 15 minutes of incubation at room temperature, whereas stabilized samples showed unchanged levels of staining even after 24 hours at room temperature prior to formalin fixation, indicating complete stabilization of phosphorylations.
The importance of correct sample preparation cannot be overstated. Sample quality is crucial and must be considered throughout the whole experiment. The Stabilizor system eliminates degradation and is compatible with all major protein study workflows. In addition, it enables preservation of the proteome in a biologically relevant state enabling confident biomarker identification.
Mats Borén, Ph.D. ([email protected]), is head of development at Denator.
Web: www.denator.com.