September 1, 2008 (Vol. 28, No. 15)
Martha Knight Ph.D.
Refinements in Classic Technology Assist in the Purification of Peptides and Proteins
Breakthrough technology that makes it possible to chromatograph larger molecules including proteins and peptides was recently licensed from the NIH by CC Biotech. The new technology encompasses spiral rotors for countercurrent chromatography (CCC), which improves on the abilities of planetary centrifuges.
The first rotor tested that was able to successfully separate proteins was a 17.5 cm OD spiral disk assembly of eight Kel-F plates separated with Teflon sheeting and clamped between aluminum alloy flanges. The spiral disk assembly of eight stacked disks has a single clockwise spiral flow channel from center to periphery with a narrow return channel underneath.
In a planetary centrifuge, in-flow and out-flow tubing enters the central axis through an opening in the top. The tubing, which is clamped to avoid twisting, passes through the central solar axis and exits from the bottom to enter the separation rotor (planetary) axis.
The spiral rotor, displaced from the central axis of rotation, completes two rotations in one revolution around the central axis, which twists and untwists the flow tubing. The planetary centrifuge apparatus functions as a separation unit in a LC system. A valve manifold has a sample loop and a solvent-gas selector valve on the in-flow tubing. Solvent flow is provided by an LC pump and outflow enters a UV detector and fraction collector.
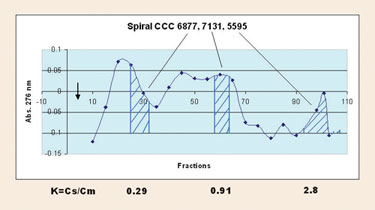
Figure 1
Peptide Separations
Once it was shown that the eight-disk rotor could be successfully used to separate peptides, additional studies were conducted with mixtures of peptides with known partition coefficients.
Separation is conducted by first selecting a solvent system where the analyte has a suitable partition coefficient of around one. The solvent system is mixed, equilibrated, and separated. The rotor in the planetary centrifuge is filled with the stationary phase, the sample is dissolved in equal volume of both phases, and then a small volume is loaded through the sample loop.
Centrifugation is then started, usually at 800 rpm, and the flow of the mobile phase is run at 1 or 2 mL/min. Fractions are collected, and the absorbance is read to determine location of the peptide. For recovery, the fractions can be directly lyophilized, or the fractions can be evaporated down in a centrifugal evaporator. The fractions are analyzed by HPLC to determine purity and identity.
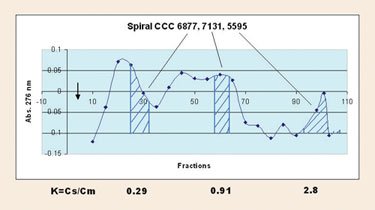
Figure 1 shows a chromatogram of a peptide mixture. HPLC analysis of the countercurrent chromatography fraction showed that the order of elution corresponded with partition coefficients and each peptide was completely separated. Since the stationary phase is the upper or organic phase, the more hydrophobic peptides were eluted later.
The spiral disk assembly has also been evaluated for preparative purification. Numerous sample loadings were made, from 10 to 85 mg, with purification resulting in all cases in the 153 mL rotor. The major component was separated from minor components. Peptides that are hydrophobic and difficult to recover from reverse-phase HPLC were recovered in high yields. For a crude peptide with dark color due to acid sensitivity of a tryptophan amino acid residue, the percent trifluoroacetic acid was reduced to 0.1%, and the lower phase was used as the mobile phase so the fractions collected could be directly lyophilized to prevent acid-catalyzed darkening of the peptide (Figure 2).
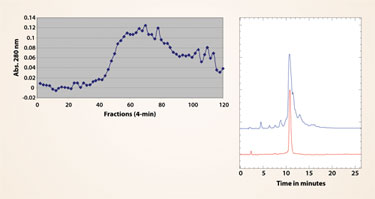
Figure 2
Nondenaturing Solvents
Spiral CCC rotors are also able to retain the stationary phase of two-phase aqueous solvent systems and as such were evaluated for the separation of proteins. In another experiment with the spiral disk assembly, it was able to separate 50 mg each of myoglobin and lysozyme. Ensuing experiments with the mixer-settler spiral disk, in which eight stacked disks have four interweaved spiral channels, showed increasing efficiency in separating model proteins.
PEG-phosphate aqueous two-phase solvent systems usually distribute PEG in the upper phase, and in most experiments the lower phase is used as the mobile phase. The CCC solvent system is nondenaturing to proteins, and thus will be useful for molecular fractionation of proteins.
At high centrifugation and flow rates proteins can be separated at high resolution. Baseline separation of the proteins at all flow rates for the runs at 1,000 rpm were obtained with high efficiency (N>1,000).
Additional progress was made with the spiral tubing support (STS) rotor (Figure 3), where the tubing was modified by pressing it to disturb the laminar flow and squeeze more layers in the rotor. This increases the tubing length. The stationary phase retention is kept high at 2 mL/min flow rate by increasing rotational speed to 1,200 rpm. The elution time is faster while good resolution is maintained indicating high-performance CCC conditions for proteins.

Figure 3a

Figure 3b
Martha Knight, Ph.D., is executive and scientific director of CC Biotech. Web: www.ccbiotech.us. Email: [email protected].