September 1, 2016 (Vol. 36, No. 15)
Duncan J. Huston-Paterson D.Phil. Integral Molecular
Soma S.R. Banik Ph.D. Integral Molecular
Benjamin J. Doranz Ph.D. President and CEO Integral Molecular
A High-Throughput Platform for Identifying Membrane Protein Antibody Targets
To discover therapeutically relevant antibodies efficiently, discovery groups are increasingly turning to functional screening strategies that can quickly isolate molecules with desired cellular effects. The challenge of this approach, however, is characterizing antibodies of interest to identify their binding targets. The lack of suitable methods to identify the targets of functional antibodies and ligands, particularly membrane proteins (which are the targets for more than 60% of FDA-approved antibody therapies), has led to freezers full of potential therapeutic candidates with no known target.
The persistence of this problem is exemplified by alemtuzumab (Campath), the first FDA-approved monoclonal antibody discovered using functional screening, which required nearly a decade of biochemical analysis before finally identifying CD52 as its target.
The Membrane Proteome Array
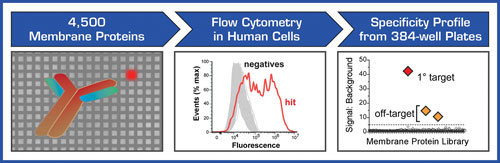
To address the need for antibody target deconvolution and receptor identification, Integral Molecular has developed the Membrane Proteome Array (MPA). The MPA is a high-throughput cell-based platform for identifying the targets of orphan antibodies and other ligands that bind to membrane proteins (Figure 1).
Membrane proteins account for roughly a quarter of all the proteins encoded by the human genome and often fold into conformationally complex structures that are difficult to retain outside of the cell. The key feature of the MPA is that human membrane proteins are individually expressed and tested in their native state directly within human cells, thereby retaining their structural integrity and native post-translational modifications.
The MPA makes use of the largest membrane protein library yet assembled, representing over 4,500 unique human membrane proteins. Each membrane protein clone in the MPA includes a C-terminal epitope tag, allowing confirmation of protein expression and the ability to verify the integrity of the membrane protein library.
Integral Molecular has leveraged its 15 years of experience working on membrane proteins by including optimized variants of the most recalcitrant membrane proteins, overcoming challenges with their expression and trafficking.
The screening process at Integral Molecular has been entirely automated, enabling rapid and reproducible binding profiles to be generated. To provide the highest level of sensitivity, the MPA uses flow cytometry for single-cell detection, enabling the elucidation of both primary and secondary binding targets. Because the MPA expresses each membrane protein directly within living cells, it can be used to identify the targets of many types of interactions that require functional readouts based on cellular activity. Besides antibody targets, the MPA can identify the receptors of natural protein ligands, toxins, and viruses.
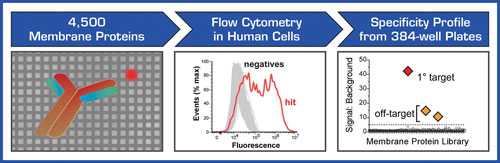
Figure 1. Integral Molecular’s MPA is a high-throughput cell-based platform for identifying the membrane protein targets of antibodies and other ligands. Membrane proteins are expressed in human cells within 384-well microplates, and ligand binding is detected by flow cytometry, allowing sensitive detection of both specific and off-target binding.
Antibody Specificity Profiling
The MPA was initially validated by testing the specificity of antibodies with known membrane protein targets. The therapeutic antibody rituximab (anti-CD20) and a lead candidate antibody against an ion channel (anti-P2X3) were tested for reactivity against the MPA (Figure 2A). The known target for each of these antibodies was readily identified, with signals 20- to 60-fold higher than background. For the anti-P2X3 candidate, the MPA also identified binding to an additional membrane protein, informing the final lead selection in this program to avoid off-target binding that could cause patient side-effects.
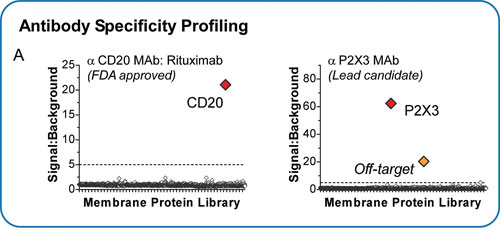
Figure 2. (A) Integral Molecular’s MPA has been used to profile the specificity of antibodies with known targets, thereby de-risking antibody development by identifying off-target binding that could cause patient side-effects.
Antibody Target Identification
In the course of developing antibodies against membrane proteins, Integral Molecular has identified hundreds of orphan antibodies that are highly reactive with human membrane proteins but whose specificities are not yet known. The MPA was used with several of these antibodies to successfully identify their molecular targets (Figure 2B). Intriguingly, several of the targets identified (e.g. CD151) are multispanning membrane proteins (that traverse the plasma membrane multiple times), which are the most difficult types of membrane proteins to express and target. These types of studies are resulting in novel therapeutic targets and new disease biomarkers.
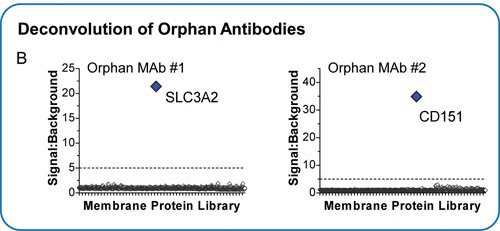
Figure 2. (B) The MPA has also been used to identify the targets of orphan antibodies, resulting in new intellectual property, novel therapeutic targets, and new disease biomarkers.
Identification of Novel Viral Receptors
The systematic array of the membrane proteome in live human cells provides a unique opportunity to test the function of each membrane protein in live cell-based assays. For example, putative cellular receptors for Zika and Ebola viruses were identified by infecting MPA-expressing nonpermissive cells with viruses that encode a fluorescent reporter protein, identifying membrane proteins that enable viral entry and infection (Figure 3). The identification of novel receptors for Zika and Ebola viruses is resulting in new intellectual property and new therapeutic strategies for combating these pathogens.
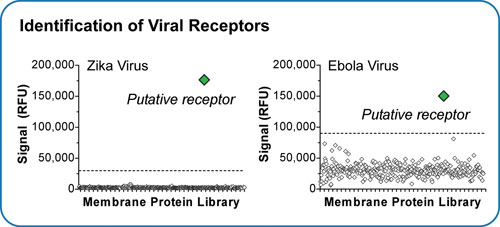
Figure 3. Integral Molecular’s Membrane Proteome Array was used to identify human membrane proteins that act as receptors for Zika and Ebola viruses. Shown is one 384-well microplate from the screening of each virus.
Conclusion
The MPA represents a rapid, comprehensive, and highly sensitive platform for identifying the membrane protein targets of a wide variety of antibodies, protein ligands, and functional interactions. The platform has been validated for use in determining target specificity, deorphaning antibodies, and identifying viral receptors, thereby de-risking lead candidate selection, establishing new intellectual property and facilitating the discovery of new disease targets.
Duncan J. Huston-Paterson, D.Phil., and Soma S.R. Banik, Ph.D., are in scientific communications, and Benjamin J. Doranz, Ph.D. ([email protected]), is president and CSO of Integral Molecular.