November 1, 2011 (Vol. 31, No. 19)
Stuart Kay
The Right Strategies Can Lower Cost of Goods and Make Field More Attractive
As highlighted in the article “Engineering Regenerative Medicine’s Future” in the September 1, 2011 issue of GEN, there is international recognition among stakeholders in the regenerative medicine sector that the commercialization of novel therapeutic interventions must be expedited in order for them to be provided by healthcare systems and providers, within the existing infrastructure.
In the last article, I wrote about standards and enabling technologies, but this time I turn my attention to approaches for reducing the total cost of goods.
Why worry about the cost of goods? Although the health economics of the different regen med therapeutic approaches are yet to be fully understood, it is generally recognized that reducing the prohibitively high costs associated with the delivery of regenerative medicine interventions is paramount to successful market access.
Of course, within the regenerative medicine space it is recognized that the diversity of therapeutic approaches (cells, devices, biomaterials, and biologics) means that there is no single economic model, and not all therapies are equal.
Nevertheless, until the costs of regen med therapies can compete with conventional biopharmaceutical therapies, where margins are typically 65–90%, the regen med industry will principally remain a niche therapy option divorced from mainstream healthcare reimbursement systems. As a result, there will be a detrimental impact on inward investment and funding into the regenerative medicine industry.
Sometimes this is because the immediate focus is on achieving a licensing deal, but potential licensors are conscious of such issues, and during due diligence will conduct thorough risk assessments and consider the balance between progressing with a constrained manufacturing approach, or the cost, time, and effort required to develop a scalable process.
How Can Engineering Help?
It is worth remembering that the majority of challenges that regen med face are no more challenging than those that are faced by the biopharmaceutical industry, and that advice can be taken from others as to how to approach some of these challenges.
Here are a few areas where product developers can focus to reduce the overall cost of goods, and produce capable and cost-effective enabling technologies and products:
-
At the early stage of the development, prepare a target product profile (TPP), which considers not only the agents with the right efficacy, but also how the therapy is to be deployed and used, and what the ideal claims would be. The TPP embodies the notion of beginning with the goal in mind.
The mode of delivery can often add an additional layer of complexity, particularly from a regulatory perspective and from the perspective of the clinical end-users. Establishing the TPP often results in a clear understanding of the regulatory pathway and the requirements of the intended delivery mechanism. This helps to minimize product waste in manufacture and reduce surgical/clinical time associated with the intervention.
This may lead to the development of a retro-injector, similar to the type used for the injection of fragile implants but designed for cell therapies. It means that the delivered load does not need to be forced beyond the end of the needle, there is lower pressure and shear on the individual cells, and there is no need to coordinate delivery with retraction.
-
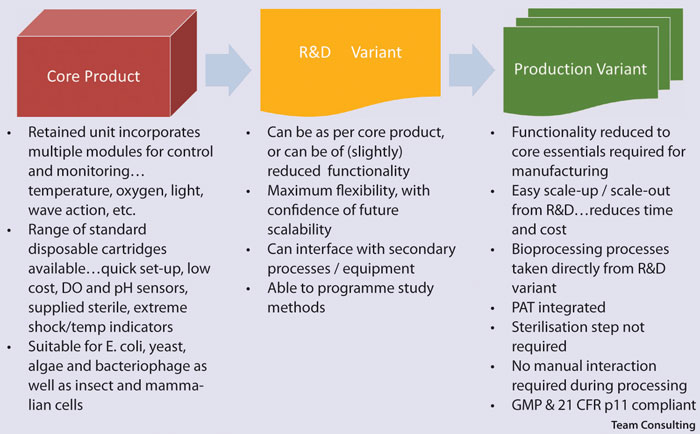
Development of modular, single-use cGxP-in-a-box systems that are appropriate for both R&D and scalable production (either scaleup or scale-out) appears especially attractive for autologous therapies, where the advantages of flexibility and relatively low volume manufacture are coupled with reduced capital spending, ease of transfer from lab to validated production, and elimination of the need to clean the system between therapies.
Given a modular design of the core product, all the surplus system functionality can be stripped out when transferring from R&D to production, thus reducing the cost even further.
-
In the previous GEN article, we discussed the need for industry collaboration to develop and introduce industry standards, in order to facilitate objective interpretation of regulatory guidance. Now consider the advantage of developing in-line testing standards, rather than batch-release standards.
Within the biopharmaceutical industry at large, this is known as process analytical technology (PAT), and it describes a fundamental shift from static batch manufacturing to a more dynamic approach. There has been a relatively slow uptake in the industry, but this is principally because it is a mature industry, and many pharma firms consider themselves locked in with their validated systems. Given that regenerative medicine is still relatively fledgling, now is the time.
The FDA describes PAT as defining the critical process parameters (CPPs) of the equipment used to make the product, which affect the critical quality attributes (CQAs) of the product and then controlling these CPPs within defined limits. This allows manufacturers to produce products with consistent quality and also helps to reduce waste and overall costs.
-
Work to ensure that the aforementioned single-use manufacturing systems and inspection systems can work together effectively. They don’t need to be integrated into a single large system, but the focus is on the avoidance of manual interaction, loss of sterility, and the need for delay due to batch release testing (Figure).
-
For those products that need shipping (which can account for 20–50% of the manufactured cost) with a reasonable amount of care—but not necessarily cold chain shipping—consider the development of a simple packing solution with low-cost disposable indicators that give a permanent and immediate indication of mishandling.
It is unlikely that all of the above will be appropriate for any single therapy, but they do demonstrate the value that engineers bring to any given development program and can help to ensure that the science can translate into robust, capable, and cost-effective product.
A further tangible benefit that will likely come as a result of such engineering work is the development and commercialisation of intellectual property (IP) relating to innovations that go beyond direct cell therapy based approaches, and which add further breadth to a developer’s overall portfolio. The European Union’s Grand Court judgement in ‘Brüstle v Greenpeace’ (October 2011), which ruled that stem cells from human embryos cannot be patented, means that the commercial value of IP in such interventions will migrate, for example, to enabling technologies such as processing equipment and devices.
Over the last decade, there have been a number of casualties in the regenerative medicine industry due to economics—sometimes due to treatment costs, and sometimes due to cash flow during extended regulatory approval time—and this has been exacerbated by the international austerity that is tightening the purse strings.
However, there are those companies and collaborations that have considered that addressing the cost of goods from the outset is critical to success, and across the realm of regen med they are typically the ones that are still surviving in these difficult times.
Addressing the complete product design, manufacture, and manner of use at an early stage means that many of the non-value-added parts and stages can be minimized or eliminated. An efficient and lean product that delivers a novel therapy, coupled with scalable manufacturing, provides an attractive investment target whether you are looking for external funding or an increased slice of internal R&D budget.
Many of the examples that I reference in this article are common problems affecting many developing regen med systems and products. By addressing the non-value-added costs, the regen med sector will be free to focus on the ground-breaking therapies being developed, which will enhance its potential to go mainstream.
Stuart Kay ([email protected]) is head of the electromechanical engineering group at Team Consulting.