May 15, 2012 (Vol. 32, No. 10)
John Russell
On paper, biosimilars have great potential to cut development costs, expand patient access to biologics, and improve affordability. The practical reality is that these goals are difficult to achieve.
Technology isn’t the biggest problem, say experts. Intellectual property challenges, added costs to characterize reference products, and uncertainty around what constitutes acceptable similarity are the stumbling blocks. That said, the biopharmaceutical industry is pushing forward aggressively to overcome the difficulties, and it’s making substantive progress.
Quality by design (QbD) approaches can reduce biosimilar development risk. Single-use technologies can cut costs. Modern bioprocessing methods produce higher titers than when originator products first reached the market. The ability to characterize large complex biologics, such as monocolonal antibodies (mAb), has improved. And the recent draft guidance from the FDA is further clarifying the regulatory landscape.
“We are all happy to have guidance documents,” says Joerg Windisch, Ph.D., head of global technical development for Sandoz Biopharmaceuticals, a player in biosimilar and innovator biologics. “They are very science-based and give FDA flexibility. We like many things about them.”
For example, the draft guidance allows global development, sparing biosimilar developers the need to conduct two clinical studies, one against the European product and one against the U.S. reference product. “We were very worried about that,” Dr. Windisch admits.
One way to help reduce development uncertainty is to use QbD concepts. “In this context, you design the manufacturing process for the biosimilar to produce a molecule that is very much the same as the original molecule,” notes Dr. Windisch.
The first step, of course, is to define the target by characterizing the reference product. This is an expensive, time-consuming process that involves buying multiple batches of the reference product and profiling the critical quality attributes (CQAs) with an arsenal of modern analytical tools.
A nagging issue is the range of variability in CQAs common to originator products including changes in the variation range over time. The next step is designing a process to produce a biosimilar that will inevitably use a different cell line, more advanced technology, and more sensitive analytics.
Sandoz employs a systematic approach, using design-of-experiment (DOE) methodologies and hundreds of experiments to characterize products and processes. Various critical attributes—basic and acidic variance, structural variation, PK/PD activities, immunogenicity, functionality, etc.—are measured and used in building mathematical models to predict process performance and product quality.
Sandoz’ work with rituximab is a good example, notes Dr. Windisch. Rituximab works largely by inducing ADCC (antibody-dependent cell-mediated cytotoxicity), which in turn is controlled by two different glycosylations on rituximab. Sandoz developed a process model that accurately predicts how much of either glycosylation occurs in response to even small changes in the process.
“We have a mechanistic model in that we have an algorithm of how different process parameters (pH, temp, addition of sugar precursors, etc.) systematically influence both of these glycosylation parameters, and we can forecast if we run a process a certain way what levels of ADCC response we will get,” he says.
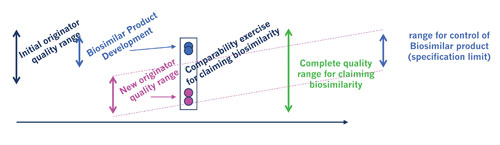
Development of a biosimilar—timeline [Sandoz Biopharmaceuticals]
Proving Biosimilarity
Processing technologies have advanced such that “you really can’t go back and recreate a cell line and expression system that would be the same as the innovator company,” says Morrey Atkinson, Ph.D., CSO and vp of R&D and drug manufacturing for Cook Pharmica.
On the upside, modern processes have higher yields. One downside is that the biosimilar’s purity profile can be quite different from the original’s purity profile. “That presents additional challenges in terms of understanding the impacts on safety and efficacy during your fairly abbreviated preclinical and clinical development. When innovator compounds came to market, there was no specification or no standard that they had to compare to,” says Dr. Atkinson.
“We know more now but we don’t yet have the ability to dial in the characteristics we want. It’s why the agencies talk about the totality of evidence when evaluating submissions.”
The challenge, explains Dr. Atkinson, is to screen clones, cell lines, and cell culture conditions that would match the reference product’s biological activity and then develop purification processes “where your acidic and basic variance and polymer levels, all of those things, are within some predefined criteria the innovator compound has established.”
Regulators struggle with the same issue. “It seems to me that EMA is trying to be more formulaic and FDA is trying to be more case-by-case. That doesn’t mean your outcome would be much different in either case. FDA is saying it wants to drive the discussion and be involved in design of the programs you develop. EMA is not doing that; it is saying these are our guidelines per class of molecule, meet these guidelines and then bring forward your application,” says Dr. Atkinson.
A surprising point in FDA’s guidance “is you don’t need to match the closure or delivery system of the current marketed product. One would think you should be close if you thought that had any effect on PK/PD. It was interesting in the FAQs they specifically say you do not need to even match the dosage form of the market product. For product people, that raises eyebrows.”
mAbs Present Twin Challenge
At 155 K or more Daltons, mAbs present a complicated challenge for biosimilar developers. Just correctly identifying the complete structure of the reference product can be daunting. Originators will not share information such as “the exact sequence and all of the post-translational modifications,” says Fiona Greer, Ph.D., global director, biopharma services development for SGS M-Scan, a provider of analytical services with biologics experience.
“We’re talking about a different scale of protein to conquer with the antibody,” explains Dr. Greer. “Smaller proteins like growth hormones and even erythropoietin—which is a bigger molecule but that’s because 30 percent of its mass is carbohydrate—are relatively straightforward in terms of physicochemical characterization.”
The second big challenge is proving that your product is sufficiently comparable to the reference product. To some extent the same analytical tools are used for each task (mass spec, peptide mapping, various chromatographic approaches, etc.) to analyze both primary and higher-order structure. De novo analysis approaches are used in determining the reference compound’s structure, keeping in mind there are always small variations among some critical attributes that regulators have deemed acceptable.
Demonstrating structural similarity is a bit different. “Actually we interrogate the two molecules (reference and biosimilar) to see if there are any differences, so we keep drilling down. On the first pass, the molecular weights might be the same. Then we might do the peptide map to spot any differences. If there aren’t any, we would we go to the next level, each time going deeper into the molecules,” said Dr. Greer.
“Physicochemical comparison is only the first step,” she stresses. “The second step would be to see if those differences impact the biological activity or safety profile. We have learned in Europe that EMA expects the primary amino acid structure to be the same to be a biosimilar—this is now clearly stated in the revised Quality Guideline. However, there can be slight differences, for example, in the carbohydrate structure or C-terminal lysine heterogeneity if this does not affect the efficacy or safety of the molecule—but this is always treated on a case-by-case basis.”
Cut Cost and Risk with SUT
Single-use technology’s (SUT) inherent flexibility and lower costs can mitigate the higher risk presented by biosimilar projects, says Bill Whitford, Ph.D., senior market manager for cell culture at Thermo Fisher Scientific.
“It can take a year or two to get a glass and steel facility going. You can obtain a disposable bioreactor, even a 2,000 liter one, within a matter of weeks and people have had them operating within hours and days of receiving them,” notes Dr. Whitford.
“The initial investment is also reduced. For example the cost of a single-use bioreactor can be 1/10 of a glass and steel welded one. It requires much less footprint and qualification of the facility. You save in square footage, in classification of the facility, and in the services required. A single-use bioreactor can operate without any purified water.”
It’s also easier to move or expand SUT operations. “Once you validate the tank and the liner for your purpose, you’ve validated the concept, and you can put a bag in another site in a different unit and all that validation transfers,” said Dr. Whitford.
Integration of Analytics Is Powerful
Echoing the importance of correctly characterizing CQAs of both reference product and biosimilar, Phil Ball, Ph.D., technical director, Eden Biodesign (part of The Watson Group), notes that the recent advances in combining the results of differing analytical methods is providing biosimilar developers with a powerful new tool.
“It’s not so much a single technology as a strategy,” he remarks. “This is a big step forward. We now use a range of highly sensitive and precise analytical methods and can combine that data to get a greater understanding of the product.”
Dr. Ball also says disposable technologies are likely to play an important role in containing costs. “We don’t believe biosimilars will completely erode the original markets, at least in the near term, and it’s likely that there will be more than one biosimilar manufacturer for any given reference product. This means that the bulk manufacturing demand and required process volumes for a single biosimilar manufacturer are likely to be less than those required by the originator company.
“This brings us in to the range where disposable manufacturing options become viable. We know that disposable technologies can provide us with improved cost of goods at these scales and also greater flexibility,” he points out. “You can install these systems in a small-scale facility with decreased capital costs, and the flexibility may allow you to produce multiple biosimilars in a single facility.”

Recent advances in combining the results of differing analytical methods is providing biosimilar developers with powerful new tools. [Eden Biodesign]
NMR for Biosimilar Process Optimization
The European Medicines Agency (EMA) approved its first biosimilar—Omnitrope, in 2006—and has now okayed 14 biosimilar products. In the U.S., the FDA recently (Feb. 9, 2012) published long-awaited draft guidelines to assist companies in the development and approval of biosimilars.
Although biosimilar manufacturers face a number of process-related obstacles, NMR profiling technology can support more effective and characterized production processes, according to Frederic Girard, Ph.D., CEO and co-founder of Spinnovation Biologics and Spinnovation Analytical. Spinnovation has developed Spedia-NMR™ technology to assist in the manufacture of biologicals and biosimilars.
The original cell line, the exact fermentation and purification process, the active drug substance, and any information relating to biosimilars are in principle not available to the follow-on manufacturer, explains Dr. Girard, adding that it is possible that copy versions of established biologics may perform differently than the original branded version. In addition differences in impurities arising from the cell culture medium or breakdown products could have serious health implications.
“Minute differences in raw materials, process, or the manufacturing environment, can result in vast differences in potential side effects caused by the final product,” says Dr. Girard. “Even two apparently similar biologics can affect the immune system in very distinct ways. This makes biosimilars distinct from small molecule generic products, and they consequently have to follow different registration pathways.”
As a result of these issues, and the FDA Pathway for Biosimilars Act (2009), the introduction of new biosimilars to the marketplace has yet to reach its full potential. Biosimilar products require a demonstration of bioequivalence between the new version and the originator product as well as a well-considered pharmacovigilance plan.
Solution with NMR
Dr. Girard maintains that NMR profiling can enhance the development of biosimilars in two ways.
“First, it can be used as a development tool to qualify cell culture media and achieve optimal conditions for biosimilars,” he points out. “Second, it can be used to examine spent media, intracellular media, or solutions from downstream processing to determine the identity and concentration of metabolites and eventual contaminants as an exercise in product safety and also to gain additional information on the newly developed ‘similar’ process.’”
Process development and optimization of cell culture media is essential to commercial viability in the production of biosimilars. Downstream factors such as scalability, performance consistency, and overall cost of manufacture can all be positively influenced by increased understanding and control of early cell growth conditions and quality of raw materials.
By understanding which factors limit cell-growth or are risk factors to low performance, developers can manipulate and monitor conditions for optimum production.
“Overall NMR profiling can provide a rapid and useful method of reverse engineering and optimizing an existing biologic process to provide a sound basis for the production of a biosimilar,” says Dr. Girard.
According to Spinnovation Biologics, its Spedia-NMR technology, which was developed to assist in the manufacture of biologicals and biosimilars, can support more effective and characterized production processes.