November 1, 2011 (Vol. 31, No. 19)
John Russell
FAIMS and Other Advances Greatly Extend Technological Boundaries
Tradeoffs between resolution, sensitivity, and speed are inevitable in chemical analysis but strides are being taken to boost resolution and to improve “qual-quant” methods that enable researchers to identify and quantify compounds in a single workflow.
Chromatography and mass spec (MS) are the staples in these efforts, but newer assistive technologies such as FAIMS and improved software are also extending the reach of these traditional tools. This was made vividly clear during a number of presentations at “Pittcon”, held in Atlanta earlier this year.
FAIMS (high-field asymmetric waveform ion mobility spectrometry) is already demonstrating higher resolution for peptides and lipids and shows great promise for applications identifying post-translational modifications (PTMs), said Alexandre Shvartsburg, Ph.D., whose lab at the Pacific Northwest National Lab, has achieved the highest resolution to date (~330 peptides) using planar FAIMS.
While not exactly new, FAIMS has undergone steady refinement with at least one commercial FAIMS product on the market and several more announced, added Dr. Shvartsburg. Broadly, FAIMS is a gas-phase, ion-separation, interface technology that permits researchers to more effectively separate ions and select which of those ions are passed to the MS for analysis.
Dr. Shvartsburg noted that, “FAIMS is not a single thing. It’s a technology. We are working on really pushing the frontier of resolution at the expense of sensitivity, unfortunately as always. Others use it differently.
“For localization of post-translational modifications, FAIMS pretty much beats any other method, and I think this is going to be a major application. So far we’ve tried it for phosphorylation and acetylation, but there is really no reason (e.g., special chemistries) it should apply only to those two.”
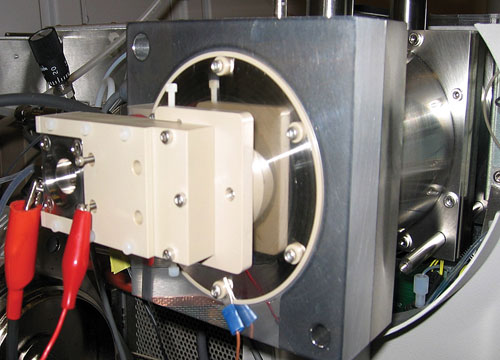
Researchers at the Pacific Northwest National Lab have achieved the highest resolution to date with planar FAIMS. A custom planar FAIMS unit is shown secured to the front end of a mass spectrometer.
Tunable Process
In FAIMS, the asymmetric waveform combines a high-strength dispersive voltage (DV), which moves ions toward one or the other electrodes, with a lower strength compensation voltage (CV) used to trap select ions in the sample and send them into the MS. An inert gas is used to shorten the time required for ions to reach terminal velocity.
The entire process is tunable (varying the CV, DV, gas concentration) so researchers can change which ions are trapped and sent to the MS. (For a complete explanation, see www.FAIMS.com.) The net result is better noise reduction, greater ion separation, and higher resolution.
FAIMS systems have varying attributes, some suited for proteomics (larger molecules) and others for metabolomics (smaller molecules). One publicly announced effort (Agilent Technologies and Owlstone) “is very unique,” said Dr. Shvartsburg. “It’s a microchip FAIMS that is almost invisible and sits within the inlet capillary to the mass spec and is smaller than the size of the fingernail. I have worked with them. They don’t have the kind of resolution we have, but they do have much better sensitivity and the speed is amazing at 10 ms analysis.”
Dr. Shvartsburg, who has published widely on FAIMS, said the technology might find use in histone code analysis, “which some people will say is the most important area of PTMs.” He expects FAIMS resolution to improve by two- to threefold over the next couple of years.
One intriguing result is FAIMS ability to distinguish isotopomers. “It’s new but FAIMS can separate some isotopomers in the gas phase. It is stunning and so far not theoretically understood. We discovered it accidentally. It could be a fundamentally new approach for identification of molecular structure using the gas phase of the molecule. In a way like NMR it fingerprints molecules in solution but the resolution would have to get much better.”
Microbial Genome Mining
Discovery of new antibiotics has slowed so much that “we could run out antibiotics to treat life-threatening infection very soon,” said senior researcher Lijiang Song, Ph.D., experimental officer in mass spectrometry at the University of Warwick. “This is why the American infectious disease society proposed that we need to discover 10 new antibiotics by 2020, which many people think is overambitious if not impossible. The main reason has been the high rate of re-discovery of known compounds.
“We have addressed this problem by employing high-resolution mass spectrometry to do de-replication in the modern microbial genome mining approach for novel natural products. This means we can identify known compounds very early on and shift our focus to true novel compounds.
“We have successfully identified and characterized more than 30 new compounds in the last five years and patented two families of novel bioactive microbial polyketide natural products (as antibiotics or anticancer agents.)”
Dr. Song’s methodology was created “to analyze the genome sequence first to identify potential interesting secondary metabolite gene clusters, such as PKS, NRPS, where one can even predict whole or part of the structure of the secondary metabolite if made.
“We then make knock-out mutants and carry out comparative metabolite profiling with LC-UV-MS/MS for wild-type and knock-out mutants to identify novel targets, followed by structure elucidation with MS and NMR.”
Dr. Song labels HR-MS/MS as “probably the most efficient way to do de-replication while high-resolution and high-accurate MS is probably the most sensitive (nanogram or even lower) and reliable way to do de-replication.”
With the emergence of high-speed, high-resolution MS, particularly the Bruker MaXis UHR-Q-TOF, said Dr. Song, it has become possible to screen a large number of samples for novel bioactive natural products by using UHPLC coupling with HR-MS/MS and still identify novel compounds quickly.
Dr. Song predicts that this approach will find its way into most natural product-based drug discovery programs. “Apparently, the current 40–60,000 resolution is good enough for most of the things we analyze, but it is not enough to distinguish sulphur isotope, which is one of the element contained in many natural products.
“I would like to think the resolution of Q-TOF HRMS will reach 100,000 in a few years time. By then HRMS/MS will become even more useful. Though many may argue that FT-ICR is better, the running cost for FT-ICR turned out to be too high for most of the labs. Their extreme high resolution (millions) is probably most suited for more specific applications.”
PK and Metabolite Identification
The ability to gather both quantitative and qualitative data in a single run using high-resolution accurate mass spectrometry coupled with UPLC for high-throughput data acquisition can substantially improve efficiency in pharma sample analysis, maintained Suma Ramagiri, Ph.D., application scientist at AB Sciex.
“For example, it’s not necessary to reanalyze the same sample two or three times by separate departments within the same company such as the bioanalytical group for quantitative information and the biotransformation group for qualitative information.”
At “Pittcon”, Dr. Ramagiri presented work demonstrating the ability to gather a buspirone PK quantitation profile in rat plasma along with identification of major metabolites over an eight-hour time period, all in single injection per time point.
The accurate mass high-resolution LC/MS assay was developed on the AB Sciex TripleTOF™ 5600 system using on-the-fly multiple mass defect filter acquisition method. Calibration curve ranging from 0.1 ng/mL–1,000 ng/mL was prepared in rat plasma and evaluated for its linearity, accuracy, and precision. Buspirone-d8 was used as an internal standard. A simple sample protein precipitation was used as sample-extraction method.
The current LC/MS/MS assay showed linearity (R>0.99) with accuracy ranging from 80–120%. Absolute quantification of parent and relative quantification of metabolites was processed by MultiQuant™ 2.0.1 software. From the same sample, both phase I and II metabolites were identified by MetabolitePilot™ 1.0 software.
Dr. Ramagiri believes this approach has wide applicability in drug R&D, including early discovery (in vitro metabolic stability and soft-spot analysis), development (in vivo PK analysis in different animal species like mouse, rat, dog, monkey), and preclinical/early clinical stages (definitive metabolite identification with quantitative capability to address metabolite safety and toxicity issues under MIST guidelines).
One challenge, noted Dr. Ramagiri, “is that more data generation with large file size creates data-storage and data-transfer problems. But with advances in computer technology, this can be controlled.”

The AB Sciex TripleTOF™ 5600 system reportedly integrates comprehensive qualitative exploration, rapid profiling, and high-resolution quantitation workflows on a single platform. It combines the highest sensitivity detection, high resolution with at least 5x better acquisition speed, and stable ~1 ppm mass accuracy over days of acquisition, according to the company.
Complex Sample Analysis
In a similar vein, complex sample analysis has long been challenging. Historically, it required two separate analytical experiments, and dealing with the large number of chromatographic and mass spec peaks is often problematic.
Lester Taylor, Ph.D., LC/MS platforms and programs manager, Agilent Technologies, said it is now possible to routinely acquire and use high-resolution accurate mass data (from unbiased full scan acquisition) for both qualitative and quantitative information. Moreover, it is not necessary to define target analytes prior to analysis.”
Dr. Taylor reviewed various approaches to the task and in particular examined the role of sophisticated software data-mining tools (molecular feature extraction, molecular formula generation, statistical differentiation and MS/MS compound libraries) to minimize time-consuming manual data review.
Working with data from a rat PK study and using the Agilent 6540 QTOF LC/MS/MS system for the quantitation of Clozapine, Norclozapine, and Clozapine-N-oxide, Dr. Taylor’s results indicated:
- LOD in rat plasma is 1 ng/mL and 1 pg on column.
- Calibration curves in rat plasma show excellent linearity (>0.995) over three orders of dynamic range.
- Assay statistics for accuracy, reproducibility, and precision were well within accepted limits.
- The concentrations of Clozapine and metabolites measured in rat plasma PK samples with good reproducibility (%RSD<5%).
- Sophisticated software processing tools aid metabolite identification.
He pointed out that qual-quant approaches such as the one used have a natural application in drug-metabolism studies where they possess the potential to speed and simplify analyses. Vendors recognize this, according to Dr. Taylor, and there is a trend “to provide robust and reliable instruments to generate accurate and precise data—and develop software tools to facilitate automated data mining, processing, and reporting.”

According to Agilent, the 6540 Ultra-High-Definition Accurate-Mass Quadrupole Time-of-Flight liquid chromatography/mass spectrometry system offers superior data quality and advanced analytical capabilities, enabling researchers to profile, identify, characterize, and quantify low molecular-weight compounds and biomolecules with confidence.
SF ICP-MS
Magnetic sector ICP-MS, both single and multicollector, is being asked to provide lower limits of detection across the periodic table and to do so with high throughput, explained Charles Douthitt, isotope ratio mass spectrometry specialist at Thermo Fisher Scientific.
“It is continuously being asked to improve the precision for measurement of isotope ratios, both at natural abundance and in the nuclear fuel cycle,” he said. “And it is being challenged to provide measurements of radionuclides, which have hitherto been the province of photon-based technologies.”
Major advances, according to Douthitt, include changes to the ICP interface which have yielded a 100-fold increase in sensitivity; improvements to the detection system that contribute to precise measurement of very weak signals; and significant developments to the handling of very small samples.
Although his talk focused on measuring radionuclides in environmental samples, the approach “can apply to any problem where one needs to measure sub ppt levels in uL sample amounts.”
Currently, sector field ICP-MS instruments are about 5–10% of the total global market for ICP-MS, according to Douthitt, and are used primarily in research settings. However adoption is expanding as researchers extend SF machine capabilities by adding chromatography (GC, LC, CE, FFF) for on-line automated sample preparation, on line isotope dilution, and laser-sampling technology.”







